Ambucaine
- CAS NO.:119-29-9
- Empirical Formula: C17H28N2O3
- Molecular Weight: 308.419
- MDL number: MFCD00864419
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
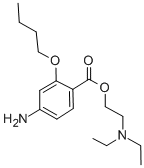
What is Ambucaine?
Originator
Ambucaine,ZYF Pharm Chemical
Manufacturing Process
A stired mixture of 4-nitro-2-hydroxybenzoic acid, anhydrous potassium
carbonate and n-butyl benzenesulfonate in xylene was refluxed under a
continuous water separator for about 19 h. The insoluble potassium salts were
filtered off and washed with hot dry toluene. The combined filtrate and
washings were distilled under reduced pressure to remove the solvents,
thereby leaving a residual oil which solidified on cooling. The solid yields of
greater than 95% of n-butyl 4-nitro-2-n-butoxybenzoate (recrystallized from
methanol).
The n-butyl 4-nitro-2-n-butoxybenzoate obtained was dissolved in 50%
aqueous ethanol. To this solution was added 2 - 3 molecular equivalents of
sodium carbonate, and the resulting mixture, was stirred under reflux for
about 16 h. After the ethanol has been distilled off under reduced pressure,
the remaining aqueous solution was diluted with water and made acidic with
concentrated hydrochloric acid. The precipitated yellow solid was filtered,
washed with water, dried in a vacuum oven at 90°C and recrystallized from
ethyl acetate. There was thus obtained a 4-nitro-2-n-butoxy-benzoic acid
(95.5% yield), melting point 120.9°-122.8°C (corr.).
A mixture of 4-nitro-2-n-butoxybenzoic acid, anhydrous potassium carbonate
and 400 ml of dry toluene was refluxed and stirred under a continuous water
separator. When the evolution of water had ceased (3 h), the water separator
was removed and there was added diethylaminoethyl chloride. The mixture
was then refluxed with stirring for about 20 h, filtered while hot, and the
solvent was removed from the filtrate by distilling in vacuo. The residual oil
was dissolved in dilute hydrochloric acid, the solution was decolorized with
activated carbon and the base was liberated by the addition of excess
ammonia. The base was extracted with ethyl acetate, the solution was dried,
and the ethyl acetate was removed by distilling in vacuo, yielding 2-
diethylaminoethyl 4-nitro-2-n-butoxybenzoate as a pale yellow oil.
The 2-diethylaminoethyl 4-nitro-2-n-butoxybenzoate in ethanol is
hydrogenated using 50 lbs. pressure of hydrogen at 25°C in the presence of
Raney nickel (alternatively platinum oxide monohydrate). After the rapid
exothermic reaction, the catalyst is filtered off and the filtrate evaporated to
dryness to give the 2-diethylaminoethyl 4-amino-2-n-butoxybenzoate.
Therapeutic Function
Local anesthetic
Safety information for Ambucaine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1