Alteconazole
- CAS NO.:93479-96-0
- Empirical Formula: C17H12Cl3N3O
- Molecular Weight: 380.65568
- MDL number: MFCD00869103
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
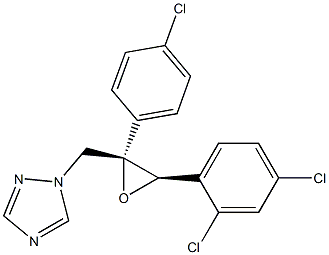
What is Alteconazole?
Originator
Alteconazole,Onbio Inc.
Manufacturing Process
63.6 g of potassium t-butylate in 300 ml of dry methanol were introduced into
a solution of 229 g of 2,4-dichlorobenzyltriphenylphosphonium chloride in 800
ml of dry methanol at 10°C, and 77.2 g of 4-chloroacetophenone were added
after half an hour. The reaction solution was refluxed for 3 hours, the
precipitated salt was filtered off at room temperature, the filtrate was
evaporated down under reduced pressure, the residue was digested with
petroleum ether at from 50°C to 70°C to free it from triphenylphosphine
oxide, and the solution was evaporated down under reduced pressure.
The residue was taken up in 1 liter of carbon tetrachloride, and the solution
was refluxed with 81.7 g of N-bromosuccinimide and 4 g of 2,2'-
azoisobutyrodinitrile. After the reaction was complete, the succinimide was
filtered off, the filtrate was evaporated down under reduced pressure and the
residue was recrystallized from methanol. 73.4 g (38.8%) of Z-1-(2,4-
dichlorophenyl)-2-(4-chlorophenyl)-3-bromoprop-1-ene of melting point 128°C
was obtained.
58.9 g of Z-1-(2,4-dichlorophenyl)-2-(4-chlorophenyl)-3-bromoprop-1-ene
were refluxed with 52.3 g of 3-choroperoxybenzoic acid in 590 ml of
chloroform. After the reaction was complete, the chloroform phase was washed acid-free with aqueous sodium bicarbonate solution and water, dried
over sodium sulfate and evaporated down under reduced pressure, and the
residue was recrystallized from methanol to give two crystalline fractions:
41.3 g (70.2%) of 2-bromomethyl-2-(4-chlorophenyl)-3-(2,4-dichlorophenyl)
oxirane (isomer A) of melting point 98-99°C, and, 12 g (20.4%) of 2-
bromomethyl-2-(4-chlorophenyl)-3-(2,4-dichlorophenyl)oxirane (isomer B) of
melting point 93-95°C.
20.9 g of 1,2,4-triazole and 4.4 g of sodium hydride (50% strength dispersion
in mineral oil) were dispersed in 150 mL of N,N-dimethylformamide, and a
solution of 39.2 g of 2-bromomethyl-2-(4-chlorophenyl)-3-(2,4-
dichlorophenyl)oxirane (isomer A) and 16.6 g of potassium iodide in 150 ml of
N,N-dimethylformamide was added at room temperature. After 8 hours, the
reaction solution was worked up, and the product was recrystallized from
diisopropyl ether. 31 g (81.9%) of 2-(1,2,4-triazol-1-ylmethyl)-2-(4-
chlorophenyl)-3-(2,4-dichlorophenyl)oxirane (isomer A) - alteconazole was
obtained. MP: 119°C.
Therapeutic Function
Antifungal
Safety information for Alteconazole
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1