Alprazolam
- CAS NO.:28981-97-7
- Empirical Formula: C17H13ClN4
- Molecular Weight: 308.76
- MDL number: MFCD00078881
- EINECS: 249-349-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
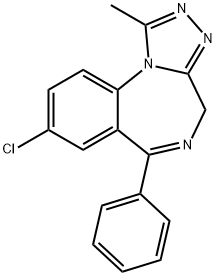
What is Alprazolam?
Absorption
Alprazolam administered orally is rapidly absorbed in the gastrointestinal tract, reaching Cmax in about 1.8 (1-2) hours. Absorption is high, resulting in an oral bioavailability of 84-91%. A 1 mg oral dose results in a Cmax of 12-22 μg/L.
The extended-release formulation of alprazolam (XANAX XR) has similar absorption, bioavailability, and pharmacokinetics as the standard release, with the exception that the Tmax is ~10 hours compared to 1-2 hours. Temporal dosing alters these parameters, with Cmax increasing by 30% and Tmax decreasing by one hour when dosed at night as opposed to in the morning.
Food has an effect on alprazolam absorption; a high-fat meal up to two hours before dosing increases the Cmax by ~25% and either a reduction (food consumed immediately prior to dosing) or increase (food consumed after dosing) of ~1/3 in Tmax. Neither the AUC nor half-life are appreciably affected by eating.
Toxicity
Alprazolam overdose can present as sleepiness, confusion, poor coordination, slow reflexes, coma, and death. Taking alprazolam with alcohol lowers the threshold for overdose. Patients should have their respiration, pulse, and blood pressure monitored. Patients can be treated by gastric lavage and intravenous fluids.. If hypotension occurs, patients may be treated with vasopressors. In known, or suspected overdoses, patients can be given the benzodiazepine receptor antagonist flumazenil in addition to other methods of management.
Oral LD50 in rats is 331-2171mg/kg.
Description
You may not have heard of alprazolam, but you may know it by its trade name, Xanax. It’s a psychoactive drug most commonly used to treat anxiety and panic disorders.
In 1970, J. B. Hester of the Upjohn Co. (now part of Pfizer) was awarded a German patent for alprazolam; the corresponding US patent was issued in 1976. Its sales took off within 2 years, and Xanax is now the most prescribed (and most abused) benzodiazepine in the United States. There is a small risk of dependence among patients with prescriptions for Xanax, but the risk is much greater for those who use it recreationally. People addicted to Xanax usually have a history of alcoholism and dependence on other drugs.
Description
Alprazolam (Item No. 14255) is an analytical reference material categorized as a benzodiazepine. Alprazolam is regulated as a Schedule IV compound in the United States. This product is intended for research and forensic applications.
Chemical properties
White Crystalline Solid
Originator
Xanax ,Upjohn ,US ,1981
The Uses of Alprazolam
Atorvastatin metabolite
The Uses of Alprazolam
Controlled substance (depressant). Anxiolytic
Indications
Alprazolam is indicated for the acute treatment of generalized anxiety disorder in adults. Alprazolam is also indicated, either as a standard or extended-release formulation, for the treatment of panic disorder with or without agoraphobia in adults.
Alprazolam may also be prescribed off-label for insomnia, premenstrual syndrome, and depression.
Background
Alprazolam is a triazolobenzodiazepine indicated for the treatment of anxiety and panic disorders. It is mainly metabolized by CYP3As and so is contraindicated with CYP3A inhibitors like ketoconazole and itraconazole. Benzodiazepine treatment should be stopped gradually by tapering down a patient's dose to avoid withdrawal symptoms. Alprazolam's adverse effects are generally related to the sedation it can cause. Alprazolam has been mixed with alcohol as a drug of abuse to potentiate the sedative effects of the drug which may lead to coma and death. Alprazolam was given FDA approval on October 16, 1981.
Definition
ChEBI: A member of the class of triazolobenzodiazepines that is 4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine carrying methyl, phenyl and chloro substituents at positions 1, 6 and 8 respectively. Alprazolam is only found in individu ls that have taken this drug.
Manufacturing Process
6-Chloro-2-hydrazino-4-phenylquinoline: A stirred mixture of 2,6-dichloro-4-
phenylquinoline (2.7 g, 0.01 mol) and hydrazine hydrate (6.8 g) was refluxed
under nitrogen for 1 hour and concentrated in vacuum. The residue was
suspended in warm water, and the solid was collected by filtration, dried and
recrystallized from ethyl acetate-Skelly B hexanes to give 1.81 g (67% yield)
of 6-chloro-2-hydrazino-4-phenylquinoline of melting point 156.5-157°C.
7-Chloro-1-methyl-5-phenyl-s-trizolo[4,3-a]quinoline: A stirred mixture of 6-
chloro-2-hydrazino-4-phenylquinoline (1.4 g, 0.0052 mol), triethylorthoacetate
(0.925 g, 0.0057 mol) and xylene (100 ml) was refluxed, under
nitrogen, for 2 hours 40 minutes. During this period the ethanol formed in the
reaction was removed by distillation through a short, glass helix-packed
column. The mixture was concentrated to dryness in vacuum and the residue
was crystallized from methanol-ethyl acetate to give: 1.28 g of 7-chloro-1-
methyl-5-phenyl-s-triazolo[4,3-a]quinoline (83.9% yield). The analytical
sample was crystallized from methylene chloride:methanol and had a melting
point 252.5-253.5°C.
5-Chloro-2-(3-methyl-4H-1,2,4-triazol-4-yl)benzophenone (Oxidation of 7-
chloro-1-methyl-5-phenyl-s-trizolo[4,3-a]quinoline): A stirred suspension of 7-
chloro-1-methyl-5-phenyl-s-triazolo[4,3-a] quinoline (2,94 g, 0.01 mol) in
acetone (110 ml) was cooled in an ice-bath and treated slowly with a solution
prepared by adding sodium periodate (2 g) to a stirred suspension of
ruthenium dioxide (200 mg) in water (35 ml). The mixture became dark.
Additional sodium periodate (8 g) was added during the next 15 minutes. The
ice-bath was removed and the mixture was stirred for 45 minutes. Additional
sodium periodate (4 g) was added and the mixture was stirred at ambient
temperature for 18 hours and filtered. The solid was washed with acetone and
the combined filtrate was concentrated in vacuum. The residue was suspended
in water and extracted with methylene chloride. The extract was dried over
anhydrous potassium carbonate and concentrated. The residue was
chromatographed on silica gel (100 g) with 10% methanol and 90% ethyl
acetate; 50 ml fractions were collected. The product was eluted in fractions
10-20 and was crystallized from ethyl acetate to give: 0.405 g of melting point 168-169.5°C and 0.291 g of melting point 167.5-169°C (23.4% yield) of
5-chloro-2-(3-methyl-4H-1,2,4-triazol-4-yl)benzophenone. The analytical
sample had a melting point of 168°C.
5-Chloro-2-[3-(hydroxymethyl)-5-methyl-4H-1,2,4-triazol-4-yl]benzophenone:
A stirred mixture of 5-chloro-2-(3-methyl-4H-1,2,4-triazolo-4-
yl)benzophenone, (2.98 g, 0.01 mol) paraformaldehyde (3 g) and xylene (100
ml) was warmed under nitrogen, in a bath maintained at 125°C for 7 hours.
The mixture was then concentrated in vacuum. The residue was
chromatographed on silica gel (150 g) with 3% methanol-97% chloroform.
Fifty ml fractions were collected. The product was eluted in fractions 20-44.
The fractions were concentrated and the residue was crystallized from
ethanol-ethyl acetate to give: 1.64 g of melting point 138-142°C; 0.316 g of
melting point 138.5-141°C; 0.431 g of melting point 139-141°C (72.8% yield)
of 5-chloro-2-[3-(hydroxymethyl)-5-methyl-4H-1,2,4-triazol-4-
yl]benzophenone. The analytical sample had a melting point of 138-139°C.
5-Chloro-2-[3-(bromomethyl)-5-methyl-4H-1,2,4-triazol-4-yl]-benzophenone:
A solution of 5-chloro-2-[3-(hydroxymethyl)-5-methyl-4H-1,2,4-triazol-4-yl]-
benzophenone (328 mg, 0.001 mol) in dry, hydrocarbon-stabilized chloroform
(5 ml) was cooled in an ice-bath and treated with phosphorus tribromide (0.1
ml). The colorless solution was kept in the ice-bath for 55 minutes, at ambient
temperature (22-24°C), for 5 hours. The resulting yellow solution was poured
into a mixture of ice and dilute sodium bicarbonate. This mixture was
extracted with chloroform. The extract was washed with brine, dried over
anhydrous magnesium sulfate and concentrated. The residue was crystallized
from methylene chloride-ethyl acetate to give: 0.285 g of melting point 200-
240°C (decomposition) and 0.030 g of melting point 200-220°C
(decomposition) of 5-chloro-2-[3-(bromomethyl)-5-methyl-4H-1,2,4-triazol-4-
yl]benzophenone. The analytical sample had a melting point of 200-240°C.
8-Chloro-1-methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine: A
stirred suspension of 5-chloro-2-[3-(bromomethyl)-5-methyl-4H-1,2,4-triazol-
4-yl]-benzophenone (391 mg, 0.001 mol) in tetrahydrofuran (15 ml) was
cooled in an ice-bath and treated with a saturated solution of ammonia in
methanol (12.5 ml). The resulting solution was allowed to warm to ambient
temperature and stand for 24 hours. It was then concentrated in vacuum. The
residue was suspended in water, treated with a little sodium bicarbonate and
extracted with methylene chloride. The extract was washed with brine, dried
with anhydrous potassium carbonate and concentrated. The residue was
crystallized from methylene chloride-ethyl acetate to give 0.220 g of crude
product of melting point 227-228.5°C. Recrystallization of this material from
ethyl acetate gave 0.142 g of melting point 228-229.5°C of 8-chloro-1-
methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine.
brand name
Niravam (Schwarz Pharma); Xanax (Pharmacia & Upjohn).
Therapeutic Function
Tranquilizer
General Description
Alprazolam, 8-chloro-1-methyl-6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepine (Xanax), israpidly absorbed from the GI tract. Protein binding is lower(~70%) than with most benzodiazepines because of itslower lipophilicity.α-Hydroxylation of the methyl group tothe methyl alcohol (a reaction analogous to benzylic hydroxylation)followed by conjugation is rapid; consequently,the duration of action is short. The drug is a highlypotent anxiolytic on a milligram basis.
Pharmacokinetics
Alprazolam is a benzodiazepine that binds γ-aminobutyric acid (GABA) type-A receptors (GABAARs) to enhance their inhibitory effect on neurotransmission, specifically in the brain. Concomitant use with opioids may result in profound sedation, respiratory depression, coma, and death; patients taking benzodiazepines and opioids concurrently may require lower doses of one or both medications, depending on their clinical situation. Patients with pre-existing impaired respiratory function are at increased risk of adverse effects including death during treatment with benzodiazepines. In addition, due to its CNS depressant effects, patients taking alprazolam should avoid operating heavy machinery or driving and should avoid other CNS depressants such as alcohol. As with other benzodiazepines, alprazolam carries a risk of abuse, misuse, and addiction, which is higher in predisposed individuals and may require strict monitoring. Cessation of therapy may result in acute or protracted withdrawal symptoms, which may be life-threatening; the patient dose should be gradually tapered whenever discontinuation or reduced dosage are necessary. Newborns born to mothers using alprazolam later in pregnancy may suffer from sedation and withdrawal symptoms. As CYP3A is required for the initial step in alprazolam metabolism, alprazolam is contraindicated in patients taking strong CYP3A inhibitors, such as ketoconazole and itraconazole; milder CYP3A inhibitors still necessitate alprazolam dosage adjustments. Lastly, benzodiazepines may have negative effects, such as panic disorders, increased suicide incidence, and episodes of mania/hypomania, in patients suffering from depression.
Metabolism
Alprazolam is metabolized to less effective metabolites by various CYPs including CYP3A4, CYP3A5, CYP3A7, and CYP2C9. The majority of alprazolam metabolism is mediated by hydroxylation via CYP3As. 4-hydroxyalprazolam has 20% the binding affinity of the parent drug, alpha-hydroxyalprazolam has 66% the affinity, and the benzophenone metabolite has <1% the affinity.
Properties of Alprazolam
| Melting point: | 228-228.50C |
| Boiling point: | 463.51°C (rough estimate) |
| Density | 1.2000 (rough estimate) |
| refractive index | 1.6110 (estimate) |
| Flash point: | 11 °C |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble |
| pka | 2.37±0.40(Predicted) |
| Water Solubility | 70mg/L(ambient temperature) |
| CAS DataBase Reference | 28981-97-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Alprazolam(28981-97-7) |
| EPA Substance Registry System | Alprazolam (28981-97-7) |
Safety information for Alprazolam
Computed Descriptors for Alprazolam
Alprazolam manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1