ALPHA-ENDORPHIN
- CAS NO.:59004-96-5
- Empirical Formula: C77H120N18O26S
- Molecular Weight: 1745.95
- MDL number: MFCD00076381
- EINECS: 262-330-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
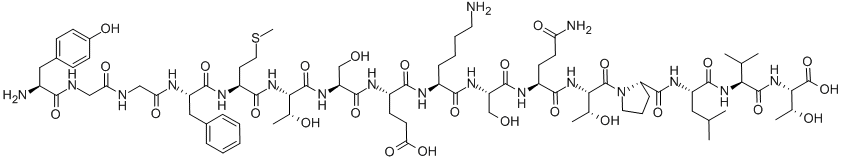
What is ALPHA-ENDORPHIN?
The Uses of ALPHA-ENDORPHIN
An opioid peptide and substrate for transglutaminase
What are the applications of Application
α-Endorphin is an endogenous opioid peptide
Biological Activity
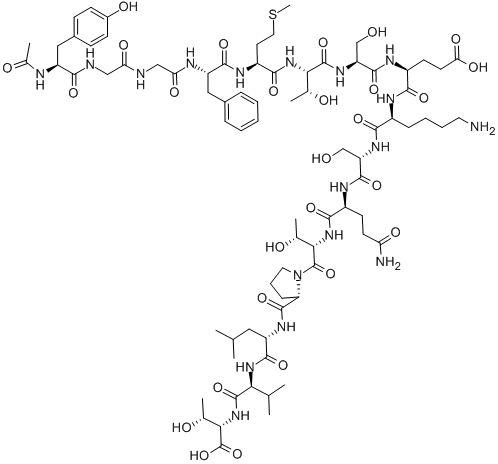
endorphins are endogenous opioid peptides that function as neurotransmitters(1). they are produced by the pituitary gland and the hypothalamus in vertebrates during exercise, excitement, pain, consumption of spicy food, love and orgasm, and they resemble the opiates in their abilities to produce analgesia and a feeling of well-being.the term implies a pharmacological activity (analogous to the activity of the corticosteroid category of biochemicals) as opposed to a specific chemical formulation. it consists of two parts: endo- and -orphin; these are short forms of the words endogenous and morphine(2), beta-endorphin (β-endorphin) is released into blood from the pituitary gland and into the spinal cord and brain from hypothalamic neurons. the β-endorphin that is released into the blood cannot enter the brain in large quantities because of the blood–brain barrier, so the physiological importance of the β-endorphin that can be measured in the blood is far from clear. β-endorphin is a cleavage product of pro-opiomelanocortin (pomc), which is also the precursor hormone for adrenocorticotrophic hormone (acth). the behavioural effects of β-endorphin are exerted by its actions in the brain and spinal cord, and it is presumed that the hypothalamic neurons are the major source of β-endorphin at these sites. in situations where the level of acth is increased (e.g., cushing’s syndrome), the level of endorphins also increases slightly(3).figure1 formula of a-endorphinfigure 2 mechanism of endorphin to inhibit lhrh release
References
1. Oswald Steward: Functional neuroscience (2000), page 116.2. Goldstein A, Lowery PJ (September 1975). "Effect of the opiate antagonist naloxone on body temperature in rats". Life Sciences 17 (6): 927–31.3. Simantov R, Snyder S (1976). "Morphine-like peptides in mammalian brain: isolation, structure elucidation, and interactions with the opiate receptor". Proc Natl Acad Sci USA 73 (7): 2515–9.
Properties of ALPHA-ENDORPHIN
| RTECS | JZ2270000 |
| storage temp. | −20°C |
| solubility | ≥174.5 mg/mL in DMSO; insoluble in EtOH; ≥52.7 mg/mL in H2O |
| form | White powder |
| Stability: | Hygroscopic |
Safety information for ALPHA-ENDORPHIN
Computed Descriptors for ALPHA-ENDORPHIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8