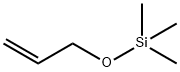
Allyltrimethylsilane
Synonym(s):3-(Trimethylsilyl)propene
- CAS NO.:762-72-1
- Empirical Formula: C6H14Si
- Molecular Weight: 114.26
- MDL number: MFCD00008635
- EINECS: 212-104-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 17:50:00
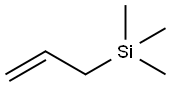
What is Allyltrimethylsilane?
Chemical properties
clear colourless liquid
The Uses of Allyltrimethylsilane
Allyltrimethylsilane is a general reagent to introduce allyl groups across acid chlorides, aldehydes, ketones, iminium ions, enones, and for cross-coupling with other carbon electrophiles. It is used as a reagent in Hosomi?Sakurai reaction.
What are the applications of Application
Allyltrimethylsilane is a trialkyl silane compound for allylation
Preparation
Allyltrimethylsilane is synthesized by the reaction of trimethylchlorosilane and allylmagnesium bromide.
What are the applications of Application
Allyltrimethylsilane is used in the allylation of aldehydes, imines, allylic and benzylic alcohols, and chiral α-keto-amides that are derived from (S)-proline esters.
Reactions
Allyltrimethylsilane is involved as a reactant in Hosomi Sakurai reaction for allylation in the presence of Lewis acid. For example, it reacts with cyclohexanone to get 1-allylcyclohexanol. It acts as a nucleophile and is involved in Carbon-Ferrier rearrangement.
As a Carbon Nucleophile in Lewis Acid-Catalyzed Reactions.
Allyltrimethylsilane is an alkene some 10 times more nucleophilic than propene, as judged by its reactions with diarylmethyl cations.It reacts with a variety of cationic carbon electrophiles, usually prepared by coordination of a Lewis acid to a functional group, but also by chemical or electrochemical oxidation,or by irradiation in the presence of 9,10- dicyanoanthracene.
carbon to give an intermediate cation, and the silyl group is lost to create a double bond at the other terminus. Among the more straightforward electrophiles are acid chlorides (eq 1).
As a Carbon Nucleophile in Fluoride Ion-Catalyzed Reactions.
The reactions with aldehydes, ketones (eq 23),54 and α,β-unsaturated esters (eq 24)55 can also be catalyzed by fluoride ion, usually introduced as tetra-n-butylammonium fluoride (TBAF), or other silicophilic ions such as alkoxide. These reactions produce silyl ether intermediates, which are usually hydrolyzed before workup. The stereochemistry of attack on chiral ketones can sometimes be different for the Lewis acid- and fluoride ion-catalyzed reactions.
Other Reactions.
Allyltrimethylsilane reacts with some highly electrophilic alkenes, carbonyl compounds, azo compounds, and singlet oxygen to a greater or lesser extent in ene reactions that do not involve the loss of the silyl group, and hence give vinylsilanes in a solvent-dependent reaction.
Flammability and Explosibility
Flammable
Purification Methods
Fractionate it through an efficient column at atmospheric pressure. If impure, dissolve it in THF, shake it with H2O (2x), dry (Na2SO4), filter and fractionate it. [Cudlin & Chvalovsky′ Collect Czech Chem Commun 27 1658 1962, Beilstein 4 IV 3927.]
Properties of Allyltrimethylsilane
| Boiling point: | 84-88 °C (lit.) |
| Density | 0.719 g/mL at 25 °C (lit.) |
| vapor pressure | 82hPa at 25℃ |
| refractive index | n |
| Flash point: | 45 °F |
| storage temp. | 2-8°C |
| solubility | freely sol all organic solvents. |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.72 |
| Water Solubility | insoluble |
| Hydrolytic Sensitivity | 2: reacts with aqueous acid |
| BRN | 906755 |
| Stability: | Volatile |
| CAS DataBase Reference | 762-72-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Silane, trimethyl-2-propenyl-(762-72-1) |
| EPA Substance Registry System | Silane, trimethyl-2-propenyl- (762-72-1) |
Safety information for Allyltrimethylsilane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Allyltrimethylsilane
| InChIKey | HYWCXWRMUZYRPH-UHFFFAOYSA-N |
Allyltrimethylsilane manufacturer
Covalent Incorporation
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 762-72-1 Allyl Trimethyl Silane 98%View Details
762-72-1 Allyl Trimethyl Silane 98%View Details
762-72-1 -
 792-72-1 98%View Details
792-72-1 98%View Details
792-72-1 -
 Allyl trimethyl silane 98%View Details
Allyl trimethyl silane 98%View Details
762-72-1 -
 Allyltrimethylsilane CAS 762-72-1View Details
Allyltrimethylsilane CAS 762-72-1View Details
762-72-1 -
 Allyl Trimethyl Silane CAS 762-72-1View Details
Allyl Trimethyl Silane CAS 762-72-1View Details
762-72-1 -
 Allyltrimethylsilane CAS 762-72-1View Details
Allyltrimethylsilane CAS 762-72-1View Details
762-72-1 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8