Acoxatrine
- CAS NO.:748-44-7
- Empirical Formula: C23H28N2O3
- Molecular Weight: 380.485
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
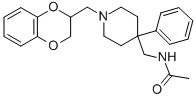
What is Acoxatrine?
Originator
Acoxatrine ,ZYF Pharm Chemical
Manufacturing Process
To a solution of 11.5 parts lithium aluminum hydride in 100 parts
tetrahydrofurane is added dropwise a solution of 94 parts DL-1-cyano-4-
phenyl-piperidine in 240 parts tetrahydrofurane, at a temperature of about
45°C. After the addition is complete, the reaction mixture is stirred first at the
same temperature for 3 h and 30 min and then refluxed for 1 h. The whole is
decomposed by successive addition of 12 parts water, 9 parts sodium hydroxide 20% and 50 parts water. The mixture is filtered from inorganic
matter. The filter-cake is washed with tetrahydrofurane and the combined
filtrates are evaporated. The oily residue is dissolved in 240 parts 2-propanol
and to this solution are added about 60 parts concentrated hydrochloric acid.
After keeping at room temperature, the precipitated salt is filtered off, washed
with 2-propanol and dried, yielding DL-4-(amino-methyl)-1-[1,4-
benzodioxanyl)methyl]-4-phenylpiperidine dihydrochloride; melting point
272°-278°C as a white amorphous powder.
From 4.1 parts DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4-
phenylpiperidine dihydrochloride, the free base is liberated in the usual
manner and extracted with chloroform. The organic layer is separated, dried
and evaporated. The DL-4-(aminomethyl)-1-[2-(1,4-benzodioxanyl)methyl]-4-
phenylpiperidine obtained is dissolved in 128 parts anhydrous chloroform. This
solution is cooled to 5°C and there is added dropwise a solution of 1.6 parts
acetylchloride in 7 parts anhydrous chloroform (exothermic reaction). The
reaction mixture is stirred over night at room temperature and then alkalized
with about 25 parts sodium hydroxide 20% at a temperature of 20°C. The
aqueous layer is separated and extracted twice with chloroform. The combined
organic layers are washed with water, dried over magnesium sulfate, filtered
and evaporated. The oily residue is dissolved in a mixture of 40 parts acetone
and 20 parts diisopropyl ether and evaporated again. The solid residue is
triturated in diisopropylether, yielding DL-4-(N-acetylaminomethyl)-1-[2-(1,4-
benzodioxanyl)-methyl]-4-phenylpiperidine; melting point 140°-141.1°C, as a
white microcrystalline powder.
Therapeutic Function
Vasodilator, Antihypertensive
Safety information for Acoxatrine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1-[2-(2-METHOXYPHENOXY)ETHYL]-PIPERIDINE](https://img.chemicalbook.in/CAS/GIF/105602-16-2.gif)
![1-[1-(2-METHOXYETHYL)PIPERIDIN-4-YL]METHANAMINE](https://img.chemicalbook.in/CAS/GIF/CB52451760.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1