Acetovanillone
Synonym(s):4′-Hydroxy-3′-methoxyacetophenone;4-Hydroxy-3-methoxyacetophenone, Acetovanillone, NADPH Oxidase Inhibitor V, NOX Inhibitor V;Acetovanillone;Apocynin;Apocynin - CAS 498-02-2 - Calbiochem
- CAS NO.:498-02-2
- Empirical Formula: C9H10O3
- Molecular Weight: 166.17
- MDL number: MFCD00008747
- EINECS: 207-854-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
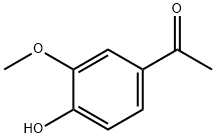
What is Acetovanillone?
Chemical properties
Acetovanillone is a yellowish to brown powder, soluble in alcohol, essential oils, and perfume chemicals. It is slightly soluble in water at room temperature but readily dissolves in hot water. It has a very faint, sweet odor, faintly reminiscent of vanillin, but with less spiciness and somewhat fresher notes. It occurs widely in nature, including in Orris root distillate.
The Uses of Acetovanillone
Acetovanillone is an inhibitor of NADPH oxidase (an enzyme responsible for reactive oxygen species production) and is useful in the treatment of various inflammatory diseases.
The Uses of Acetovanillone
4'-Hydroxy-3'-methoxyacetophenone is used as an inhibitor of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which is an enzyme responsible for reactive oxygen species production. It is also useful in the treatment of various inflammatory diseases and atherosclerosis. Further, it shows an anti-inflammatory activity and improves endothelial function by reducing oxidative stress. In addition to this, it is used for studying nitric oxide regulated processes in plants.
What are the applications of Application
Apocynin is a methoxy-substituted catechol commonly used as an inhibitor of NADPH oxidase
Definition
ChEBI: Apocynin is an aromatic ketone that is 1-phenylethanone substituted by a hydroxy group at position 4 and a methoxy group at position 3. It has a role as a non-narcotic analgesic, a non-steroidal anti-inflammatory drug, an antirheumatic drug, a peripheral nervous system drug, an EC 1.6.3.1. [NAD(P)H oxidase (H2O2-forming)] inhibitor and a plant metabolite. It is a member of acetophenones, a methyl ketone and an aromatic ketone.
Preparation
Synthetically by methylation of 3,4-Dihydroxyacetophenone. It can also be obtained by Fries rearrangement of guaiacol acetate in the presence of zinc chloride. It can be isolated from the extract of the roots of Indian hemp, Apocynum cannabinum L.
Production Methods
Synthetically by methylation of 3,4- Dihydroxy acetophenone. Also from Guaiacol acetate with Zinc chloride and Acetic anhydride. Can be isolated from the extract of the roots of Indian hemp, Apocynum cannabinum L.
Synthesis Reference(s)
The Journal of Organic Chemistry, 60, p. 739, 1995 DOI: 10.1021/jo00108a046
General Description
Acetovanillone is an aromatic plant ketone, which is a selective inhibitor of NADPH oxidase. It is mainly secreted in the urine of mammals as the corresponding glucuronide conjugate.
storage
Store at RT
Purification Methods
Crystallise apocynin from water, or EtOH/pet ether. [Beilstein 8 IV 1814.]
References
[1] GARA N DEXTER. Bacterial catabolism of acetovanillone, a lignin-derived compound.[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022: e2213450119. DOI:10.1073/pnas.2213450119.
[2] YUDAI HIGUCHI. The Catabolic System of Acetovanillone and Acetosyringone in Sphingobium sp. Strain SYK-6 Useful for Upgrading Aromatic Compounds Obtained through Chemical Lignin Depolymerization.[J]. Applied and Environmental Microbiology, 2022, 88 16: e0072422. DOI:10.1128/aem.00724-22.
[3] CHIARA RIGANTI. The NADPH oxidase inhibitor apocynin (acetovanillone) induces oxidative stress[J]. Toxicology and applied pharmacology, 2006, 212 3: Pages 179-187. DOI:10.1016/j.taap.2005.07.011.
[4] GJERTSEN FB Scheline R Solheim E. Metabolism of aromatic plant ketones in rats: Acetovanillone and paeonol[J]. Journal of ethnopharmacology, 1988, 23 2: Page 342. DOI:10.1016/0378-8741(88)90035-9.
Properties of Acetovanillone
| Melting point: | 112-115 °C (lit.) |
| Boiling point: | 263-265 °C/17 mmHg (lit.) |
| Density | 1.1708 (rough estimate) |
| refractive index | 1.5101 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Acetonitrile (Slightly), Chloroform (Slightly), Ethyl Acetate (Slightly), Methan |
| pka | 8.18±0.18(Predicted) |
| form | Solid |
| color | Yellow to brown |
| Odor | at 100.00 %. faint sweet vanillin |
| Water Solubility | soluble in hot water |
| Merck | 14,741 |
| BRN | 637373 |
| CAS DataBase Reference | 498-02-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanone, 1-(4-hydroxy-3-methoxyphenyl)-(498-02-2) |
| EPA Substance Registry System | Acetovanillone (498-02-2) |
Safety information for Acetovanillone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetovanillone
Acetovanillone manufacturer
GRK RESEARCH LABORATORIES PVT LTD
AVIGYABIOSCIENCES PRIVATE LIMITED
Pratap Organics Pvt Ltd
Ralington Pharma
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 3-Nitrobenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 4'-Hydroxy-3'-methoxyacetophenone 99%View Details
4'-Hydroxy-3'-methoxyacetophenone 99%View Details
498-02-2 -
 498-02-2 4′-Hydroxy-3′-methoxyacetophenone (Acetovanillone) 98%View Details
498-02-2 4′-Hydroxy-3′-methoxyacetophenone (Acetovanillone) 98%View Details
498-02-2 -
 Acetovanillone 98% (GC) CAS 498-02-2View Details
Acetovanillone 98% (GC) CAS 498-02-2View Details
498-02-2 -
 Acetovanillone 95% CAS 498-02-2View Details
Acetovanillone 95% CAS 498-02-2View Details
498-02-2 -
 Acetovanillone 98%+View Details
Acetovanillone 98%+View Details
498-02-2 -
 Acetovanillone 98%View Details
Acetovanillone 98%View Details -
 4-Hydroxy-3-methoxy acetophenone, 98% CAS 498-02-2View Details
4-Hydroxy-3-methoxy acetophenone, 98% CAS 498-02-2View Details
498-02-2 -
 4'-Hydroxy-3'-methoxyacetophenone CAS 498-02-2View Details
4'-Hydroxy-3'-methoxyacetophenone CAS 498-02-2View Details
498-02-2