ACEPROMAZINE MALEATE
Synonym(s):2-Acetyl-10-(3-dimethylaminopropyl)phenothiazine maleate salt;Acepromazine maleate salt;Acetopromazine maleate salt
- CAS NO.:3598-37-6
- Empirical Formula: C23H26N2O5S
- Molecular Weight: 442.53
- MDL number: MFCD00082473
- EINECS: 222-748-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
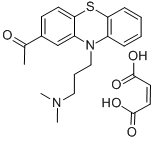
What is ACEPROMAZINE MALEATE?
Description
Acepromazine is a phenothiazine antipsychotic, analog of chlorpromazine , and a dopamine receptor antagonist. It also binds to muscarinic receptors in rat brain (IC50 = 350 nM) and inhibits contractions of guinea pig ileum induced by acetylcholine (; IC50 = 840 nM). Acepromazine inhibits morphine-induced vomiting in dogs. It also inhibits development of a conditioned avoidance response, unconditioned escape response, and catalepsy in rats (ED50s = 0.98, 3.1, and 17 mg/kg, respectively). Formulations containing acepromazine have been used as tranquilizers and anesthetics premedicant in small and large animal veterinary care.
Chemical properties
Yellow Crystalline Solid
Originator
Sedaject ,Bayer Korea Co.
The Uses of ACEPROMAZINE MALEATE
Acepromazine maleate has been used:
- as a component of anesthesia cocktail to test its effects on behavioral changes in rats
- as a component of anesthesia cocktail to study its effects on lung resistance of rats
- as an anti-psychotic pharmaceutical agent to study its cytotoxic effects on malignant glioma in glioblastoma (GBM) cells
The Uses of ACEPROMAZINE MALEATE
A tranquilizer
The Uses of ACEPROMAZINE MALEATE
antihistamine
The Uses of ACEPROMAZINE MALEATE
vitamin D2 analog, blood calcium regulator, therapeutc for chronic hypocalcemia
What are the applications of Application
Acepromazine Maleate is a dopamine inhibitor
Manufacturing Process
120 g 3-acetylphenothiazine (0.479 mol) are heated to the boil in 1.2 L xylene and a rapid stream of phosgene then passed in for a period of 12 hours. The solvent is then removed by distillation and the residue taken up in 1 L benzene. The benzene solution is heated to the boil and 112 g of γ- dimethylaminopropyl alcohol (1.09 mol) are added within a period of 15 min and the reaction mixture boiled for a further 2 hours. After cooling, the precipitated hydrochloride of the γ-dimethylaminopropyl alcohol washed with benzene and the combined benzene solutions rapidly washed with water in order to remove excess basic alcohol. The material is dried over potash and the hydrochloride precipitated by means of ethereal hydrochloric acid. The hydrochloride may also be precipitated by passing in gaseous hydrogen chloride. After recrystallisation from isopropanol, 157 g (78% of theory) of γ- dimethylaminopropyl 3-acetylphenothiazine-10-carboxylate are obtained. The hydrochloride melts at 212°C.
Therapeutic Function
Neuroleptic, Antiemetic
Biochem/physiol Actions
Acepromazine is a phenothiazine antipsychotic comonly used as a veterinary drug (horses, dogs and cats). The compound is an analogue antipsychotic drug chlorpromazine (#C8138). The drug is thought to block receptors of dopamine in the brain. Recently it was discover that the compound inhibits ABCG2 transported protein, which overexpression in tumors is associated with drug resistance.
Safety Profile
Poison by ingestion, subcutaneous,and intravenous routes. When heated to decomposition itemits highly toxic fumes of NOx and SOx.
Veterinary Drugs and Treatments
Acepromazine is approved for use in dogs, cats, and horses. Labeled
indications
for dogs and cats include: “. . . as an aid in controlling
intractable animals . . . alleviate itching as a result of skin irritation;
as an antiemetic to control vomiting associated with motion sickness”
and as a preanesthetic agent. The use of acepromazine as a
sedative/tranquilizer in the treatment of adverse behaviors in dogs
or cats has largely been supplanted by newer, effective agents that
have fewer adverse effects. Its use for sedation during travel is controversial
and many no longer recommend drug therapy for this
purpose.
In horses, acepromazine is labeled “. . . as an aid in controlling
fractious animals,” and in conjunction with local anesthesia for
various procedures and treatments. It is also commonly used in horses as a pre-anesthetic agent at very small doses to help control
behavior.
Although not approved, it is used as a tranquilizer (see doses)
in other species such as swine, cattle, rabbits, sheep and goats.
Acepromazine has also been shown to reduce the incidence of halothane-
induced malignant hyperthermia in susceptible pigs.
Properties of ACEPROMAZINE MALEATE
| Melting point: | 135-136°C |
| storage temp. | room temp |
| solubility | H2O: >10mg/mL |
| form | powder |
| pka | 9.3(at 25℃) |
| color | yellow |
Safety information for ACEPROMAZINE MALEATE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H336:Specific target organ toxicity,single exposure; Narcotic effects |
Computed Descriptors for ACEPROMAZINE MALEATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![4-[1-Chloro-2-(methylamino)ethyl]phenyl Acetate Hydrochloride](https://img.chemicalbook.in/CAS2/GIF/14593-25-0.gif)

You may like
-
 Acepromazine maleate 97% CAS 3598-37-6View Details
Acepromazine maleate 97% CAS 3598-37-6View Details
3598-37-6 -
 Acepromazine maleate CAS 3598-37-6View Details
Acepromazine maleate CAS 3598-37-6View Details
3598-37-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1