ABT450
- CAS NO.:1216941-48-8
- Empirical Formula: C40H43N7O7S
- Molecular Weight: 765.88
- MDL number: MFCD28411394
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-09-04 23:37:13
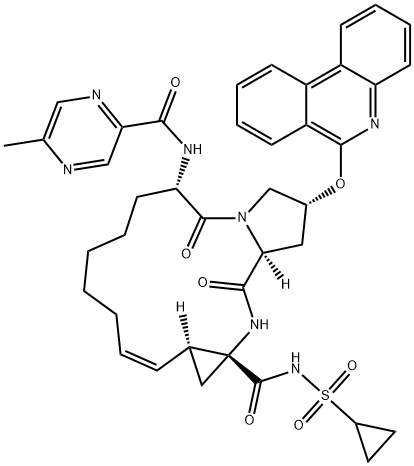
What is ABT450?
Absorption
Tmax of approximately 4 to 5 hours with a maximum concentration (Cmax) of 194 ng/mL .
Description
Paritaprevir hydrate, a second-generation NS3/4A protease inhibitor, is a component of the all-oral, interferon-free hepatitis C virus combination therapy developed by Enanta Pharmaceuticals and AbbVie. The fixed-dose tablet of paritaprevir, ombitasvir (XXV, NS4A replication complex inhibitor), and ritonavir (cytochrome P450 inhibitor) taken in combination with dasabuvir (X, NS5B polymerase inhibitor) was approved for the treatment of chronic HCV genotype 1 in the USA and EU in 2014, and further approved for treatment of genotype 4 chronic HCV infection without cirrhosis by the US FDA in 2015. After 12 weeks of combination treatment, high sustained virological response rates have been demonstrated in clinical trials.205 Paritaprevir joins other marketed NS3/4A inhibitors, including telaprevir, boceprevir, simeprevir, and vaniprevir (XXXVIII), which inhibit a critical enzymatic complex for HCV replication. It exhibits potent antiviral activity against HCV genotype 1a and 1b strains, with EC50 values of 1.0 and 0.21 nM respectively. As paritaprevir is metabolized by CYP3A4, ritonavir, a CYP3A inhibitor with no direct HCV antiviral properties, is dosed concurrently to boost paritaprevir exposure, raising the mean plasma half-life to ca. 5.5 h and allowing for once-daily dosing. While several development routes for paritaprevir have been published in the patent literature, no process route has been disclosed to date. Perceptibly the most scalable route is described below; no yields for this route have been reported. Notably, the synthesis of a closely related compound that shares the same macrocylic core has been reported by AbbVie on kilogram scale.
The Uses of ABT450
Paritaprevir is a pharmaceutical drug that is used in the treatment of hepatitis C virus in patients with HCV genotype 1 infection. It inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion.
Indications
When used within the fixed-dose combination product with Ombitasvir, Dasabuvir, and Ritonavir as the FDA-approved product Viekira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis.
When used within the fixed-dose combination product with Ombitasvir and Ritonavir as the FDA- and Health Canada-approved product Technivie, paritaprevir is indicated in combination with Ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis.
When used within the fixed-dose combination product with Ombitasvir, Dasabuvir, and Ritonavir as the Health Canada-approved, commercially available product Holkira Pak, paritaprevir is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a with or without cirrhosis.
Background
Paritaprevir is a direct acting antiviral medication used as part of combination therapy to treat chronic Hepatitis C, an infectious liver disease caused by infection with Hepatitis C Virus (HCV). HCV is a single-stranded RNA virus that is categorized into nine distinct genotypes, with genotype 1 being the most common in the United States, and affecting 72% of all chronic HCV patients . Treatment options for chronic Hepatitis C have advanced significantly since 2011, with the development of Direct Acting Antivirals (DAAs) such as paritaprevir. As a newer generation and directly acting HCV antiviral, paritaprevir products have better Sustained Virological Response (SVR) rates, higher barriers to resistance, fewer side effects, and a reduced pill burden compared to older agents such as Boceprevir, Telaprevir, Peginterferon alfa-2a, Peginterferon alfa-2b, and Ribavirin. By combining multiple antiretroviral medications into fixed dose products, the viral lifecycle can be targeted at multiple stages while simultaneously reducing the risk of developing resistant viral strains . Within Canada and the United States, paritaprevir is currently available in three fixed dose products: Viekira Pak (FDA), Technivie (FDA and Health Canada), and Holkira Pak (Health Canada).
More specifically, paritaprevir prevents viral replication by inhibiting the NS3/4A serine protease of Hepatitis C Virus (HCV) . Following viral replication of HCV genetic material and translation into a single polypeptide, Nonstructural Protein 3 (NS3) and its activating cofactor Nonstructural Protein 4A (NS4A) are responsible for cleaving genetic material into the following structural and nonstructural proteins required for assembly into mature virus: NS3, NS4A, NS4B, NS5A, and NS5B . By inhibiting viral protease NS3/4A, paritaprevir therefore prevents viral replication and function.
In a joint recommendation published in 2016, the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America (IDSA) recommend Paritaprevir as a first line therapy option when used in combination with other antivirals for genotypes 1a, 1b, and 4. Depending on the genotype, Paritaprevir is often used in combination with other antivirals such as Ombitasvir, Dasabuvir, Ritonavir, and Ribavirin, with the intent to cure, or achieve a sustained virologic response (SVR), after 12 weeks of daily therapy. SVR and eradication of HCV infection is associated with significant long-term health benefits including reduced liver-related damage, improved quality of life, reduced incidence of Hepatocellular Carcinoma, and reduced all-cause mortality . Treatment with direct acting antivirals such as paritaprevir is associated with very minimal side effects, with the most common being headache and fatigue . Lack of significant side effects and short duration of therapy is a considerable advantage over older interferon-based regimens, which were limited by infusion site reactions, reduced blood count, and neuropsychiatric effects .
Paritaprevir first came on the market as a fixed-dose combination product with Ombitasvir, Dasabuvir, and Ritonavir as the FDA-approved product Viekira Pak. First approved in December 2014, Viekira Pak is indicated for the treatment of HCV genotype 1b without cirrhosis or with compensated cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a without cirrhosis or with compensated cirrhosis.
Paritaprevir is also available as a fixed-dose combination product with Ombitasvir and Ritonavir as the FDA- and Health Canada-approved product Technivie. First approved in July 2015, Technivie is indicated in combination with Ribavirin for the treatment of patients with genotype 4 chronic hepatitis C virus (HCV) infection without cirrhosis or with compensated cirrhosis.
In Canada, paritaprevir is also available as a fixed-dose combination product with Ombitasvir, Dasabuvir, and Ritonavir as the Health Canada-approved, commercially available product Holkira Pak. First approved in January 2015, Holkira Pak is indicated for the treatment of HCV genotype 1b with or without cirrhosis, and when combined with Ribavirin for the treatment of HCV genotype 1a with or without cirrhosis.
Definition
ChEBI: An azamacrocycle which is used which is in combination with dasabuvir sodium hydrate, ombitasvir and ritonavir (under the trade name Viekira Pak) for treatment of chronic hepatitis C virus genotype 1 infection as well as cirrhosis of the liver.
Pharmacokinetics
At concentrations approximately 6 and 1.8 times the therapeutic concentrations of paritaprevir and ombitasvir, the combination did not prolong QTc to any clinically relevant extent .
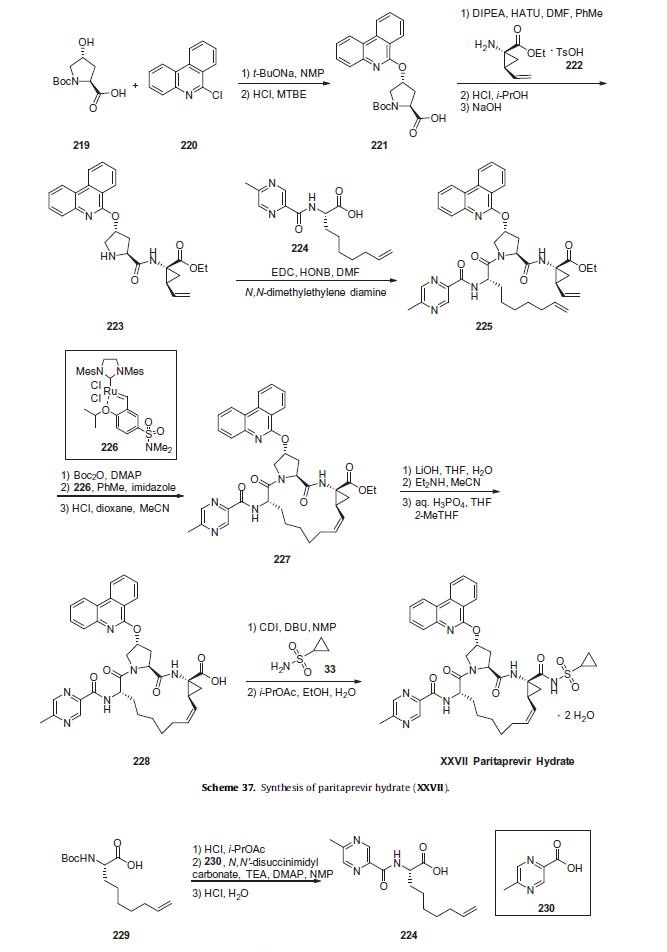
Synthesis
Commercial (2S,4R)-N-Boc-4-hydroxyproline (219)
was reacted with 6-chlorophenanthridine (220) in NMP in the
presence of sodium t-butoxide. Acid 221 was then coupled
with commercial vinylcyclopropylamine fragment 222 using
o-(7-azabenzotriazol-1-yl)-N,N,N0 ,N0-tetramethyluronium hexafluorophosphate
(HATU) and DIPEA to afford peptide 223 following
Boc deprotection. The product could be crystallized upon neutralizing
with NaOH. Amine 223 was subsequently coupled with acid
224 using EDC and N-hydroxy-5-norbornene-2,3-di-carboximide
(HONB) in the presence of N,N-dimethylethylene diamine to afford
linear tripeptide 225. Acid 224 was formed from Boc-
(2S)-amino-non-8-eic acid (229) and 5-methyl-2-pyrazine carboxylic
acid (230) via Boc deprotection and peptide coupling, using
N,N0-disuccinimidyl carbonate and 4-dimethylaminopyridine
(DMAP) to pre-activate acid 230.
Linear trieptide 225 was Boc protected and then subjected to
ring closing metathesis using Zhan-B catalyst (226) in toluene,
using imidazole to quench the catalyst after the reaction. On
kilo-scale, a closely-related ring closing metathesis reaction provided
the desired Z isomer in 61% yield.211 Removal of the Boc carbamate
then provided macrocyclic intermediate 227. Ester
hydrolysis with lithium hydroxide followed by acidification gave
acid 228 which was coupled with cyclopropylsulfonamide (33)
using CDI and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The isolated
product was dissolved in i-PrOAc and diluted with ethanol.
Water was added portion-wise and the solid isolated by filtration
to afford crystalline paritaprevir hydrate (XXVII).
Metabolism
Paritaprevir is predominantly metabolized by CYP3A4 and to a lesser extent by CYP3A5 .
Properties of ABT450
| Density | 1.45±0.1 g/cm3(Predicted) |
| storage temp. | 4°C, away from moisture and light |
| solubility | DMSO:30.0(Max Conc. mg/mL);39.2(Max Conc. mM) |
| form | A solid |
| pka | 4.41±0.60(Predicted) |
| color | White to off-white |
Safety information for ABT450
Computed Descriptors for ABT450
Abamectin manufacturer
BDR Pharmaceuticals International Pvt Ltd
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidYou may like
-
 1216941-48-8 Paritaprevir 98%View Details
1216941-48-8 Paritaprevir 98%View Details
1216941-48-8 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4