9-Borabicyclo[3.3.1]nonane
Synonym(s):9-BBN
- CAS NO.:280-64-8
- Empirical Formula: C8H15B
- Molecular Weight: 122.02
- MDL number: MFCD00074742
- EINECS: 206-000-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
![9-Borabicyclo[3.3.1]nonane Structural](https://img.chemicalbook.in/CAS/GIF/280-64-8.gif)
What is 9-Borabicyclo[3.3.1]nonane?
Description
9-BBN is typically used in organic chemistry for hydroboration-oxidation reactions. 9-BBN is a very useful reagent due to its ability to achieve high regioselectivity in reactions based on steric and electronic factors.
Chemical properties
WHITE CRYSTALLINE POWDER
The Uses of 9-Borabicyclo[3.3.1]nonane
9-BBN monomer acts as a selective hydroboration reagent in synthetic organic chemistry. It is employed in Suzuki reactions as well as in the preparation of terminal alcohols by the region selective addition of alkenes followed by oxidative cleavage using hydrogen peroxide. It is also used in copper-catalyzed cross-coupling reactions of organoboron compounds with primary alkyl halides and pseudohalides. It acts as a protecting group for alkenes. Further, it is used as a reactant for Hetero-Diels-Alder reaction for the synthesis of spirocyclic alkaloids. In addition to this, it is used in intramolecular insertion of alkenes into palladium-nitrogen bonds.
The Uses of 9-Borabicyclo[3.3.1]nonane
![9-Borabicyclo[3.3.1]nonane](/ProductImageIndia/2023-11/20231121054671543454721.png)
To a solution of the SM (500 mg, 1.82 mmol) in THF (5 mL) was added 9-BBN (0.5 M in THF, 7.30 mL, 3.65 mmol) at RT. The mixture was stirred at RT for 2 h, until no SM was detected by TLC. To the mixture at 0 C was added aq 3.0 M NaOH (0.91 mL, 2.73 mmol), followed by H2O2 (30% in H2O, 1.1 mL, 9.1 mmol). The reaction mixture was stirred at RT for 1 h, after which time it was diluted with brine (20 mL) and extracted with EtOAc (3 x 30 mL). The combined organics were dried (Na2SO4), concentrated, and purified by a flash chromatography column (1:20 to 1:3 EtOAc/PE) to provide the product as a colorless oil. [400 mg, 75%]
The Uses of 9-Borabicyclo[3.3.1]nonane
Protecting group for alkenes?
Reactant for:
- Linear SPPS synthesis of ubiquitin derivatives
- Copper-catalyzed cross-coupling reactions of organoboron compounds with primary alkyl halides and pseudohalides
- Intramolecular insertion of alkenes into palladium-nitrogen bonds
- Preparation of (phosphonoacetyl)ornithine to study effect on arginine biosynthetic genes in yeast
- Hetero-Diels-Alder reaction for synthesis of spirocyclic alkaloids
General Description
9-Borabicyclo[3.3.1]nonane is an organic compound, which is generally used as a hydroborating agent. It is thermally stable, less sensitive to oxygen and water when compared to other dialkyl boranes. It is commonly used to prepare aldehydes from alkynes.
Purification Methods
It is available as the solid dimer or in tetrahydrofuran solution. The solid is relatively stable and can be purified by distillation in a vacuum (as dimer) and by recrystallisation from tetrahydrofuran (solubility at room temperature is 9.5%, 0.78M), filter off the solid under N2, wash it with dry pentane and dry it in vacuo at ca 100o. The solid is a dimer (IR 1567cm-1), stable in air (for ca 2 months), and can be heated for 24hours at 200o in an inert atmosphere without loss of hydride activity. It is a dimer in tetrahydrofuran solution also (IR 1567cm-1). It is sensitive to H2O and air (O2) in solution. Its concentration in solution can be determined by reaction with MeOH and measuring the volume of H2 liberated, or it can be oxidised to cis-cyclooctane-1,5-diol (m 73.5-74.5o). [IR: Knights & Brown J Am Chem Soc 90 5280 1968, Brown et al. J Am Chem Soc 96 7765 1974, Brown et al. J Org Chem 41 1778 1976, Brown & Chen J Org Chem 46 3978 1981, Fieser & Fieser Reagents for Org Synth 2 31, 3 24, 10 48, 15 43, 17, 49.] Borane pyridine complex [110 -51 -0] M 92.9, m 8-10o, 10-11o, b 86o/7mm, 100-1 0 1o/12mm, d 4 0.785. Dissolve it in Et2O and wash it with H2O in which it is insoluble. Evaporate the Et2O and distil the residual oil to gives better than 99.8% purity. Its vapour pressure is less than 0.1mm at room temperature. [Taylor et al. J Am Chem Soc 77 1506 1955, Beilstein 20 IV 2235.]
Properties of 9-Borabicyclo[3.3.1]nonane
| Melting point: | 140-142 °C |
| Boiling point: | 68-70 °C |
| Density | 0.894 g/mL at 25 °C |
| Flash point: | 1 °F |
| storage temp. | Flammables area |
| solubility | Miscible with ether, hexane, benzene, toluene, carbon tetrachloride, chloroform, dimethylsulfide and dichloromethane. Slightly miscible with cyclohexane, dimethoxyethane, diglyme and dioxane. |
| appearance | Colorless solid |
| Water Solubility | reacts |
| Sensitive | Air & Moisture Sensitive |
| BRN | 605509 |
| Exposure limits | ACGIH: TWA 50 ppm; STEL 100 ppm (Skin) OSHA: TWA 200 ppm(590 mg/m3) NIOSH: IDLH 2000 ppm; TWA 200 ppm(590 mg/m3); STEL 250 ppm(735 mg/m3) |
| EPA Substance Registry System | 9-Borabicyclo[3.3.1]nonane (280-64-8) |
Safety information for 9-Borabicyclo[3.3.1]nonane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H260:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H261:Substances And Mixtures Which, In Contact With Water,Emit Flammable Gases H302:Acute toxicity,oral H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H333:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H351:Carcinogenicity H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P223:Keep away from any possible contact with water, because of violent reaction and possible flash fire. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P231+P232:Handle under inert gas. Protect from moisture. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P370+P378:In case of fire: Use … for extinction. P405:Store locked up. P402+P404:Store in a dry place. Store in a closed container. P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for 9-Borabicyclo[3.3.1]nonane
| InChIKey | FEJUGLKDZJDVFY-OCAPTIKFSA-N |
9-Borabicyclo[3.3.1]nonane manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![Bis-9-borabicyclo[3.3.1]nonane ,9-BORABICYCLO[3.3.1]NONANE DIMER,9-BORABICYCLO[3.3.1]NONANE,9-BORABICYCLO[3.3.1]NONANE,9-BORABICYCLO[3.3.1]NONANE DIMER](https://img.chemicalbook.in/CAS/GIF/21205-91-4.gif)
![LITHIUM B-ISOPINOCAMPHEYL-9-BORABICYCLO[3.3.1]NONYL HYDRIDE](https://img.chemicalbook.in/CAS/GIF/100013-07-8.gif)
![9-BORABICYCLO [3.3.1] NONANE [B-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB6774145.gif)
![B-ISOPINOCAMPHEYL-9-BORABICYCLO[3.3.1]NONANE](https://img.chemicalbook.in/CAS/GIF/42371-63-1.gif)
![Lithium 9-borabicyclo[3.3.1]nonane hydride](https://img.chemicalbook.in/CAS/GIF/76448-08-3.gif)
You may like
-
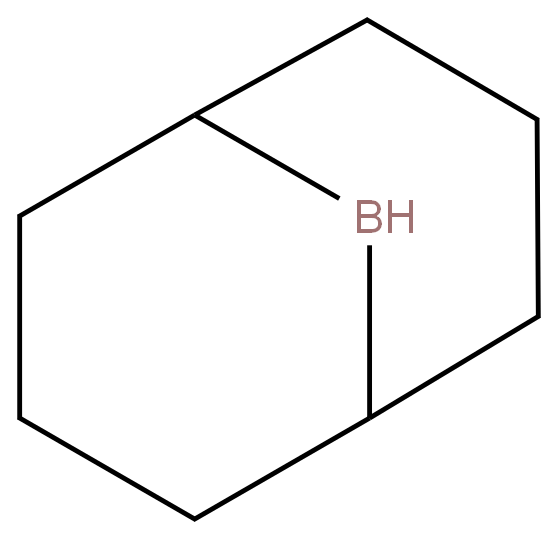 280-64-8 9-borobicyclo (3,3,1)none 0.5M in THF 99%View Details
280-64-8 9-borobicyclo (3,3,1)none 0.5M in THF 99%View Details
280-64-8 -
![280-64-8 9-Borabicyclo[3.3.1]nonane (0.5 M in THF)](https://img.chemicalbook.in//Content/image/CP5.jpg) 280-64-8 9-Borabicyclo[3.3.1]nonane (0.5 M in THF)View Details
280-64-8 9-Borabicyclo[3.3.1]nonane (0.5 M in THF)View Details
280-64-8 -
![280-64-8 9-Borabicyclo[3.3.1]nonane, 0.5M in
THF 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 280-64-8 9-Borabicyclo[3.3.1]nonane, 0.5M in THF 99%View Details
280-64-8 9-Borabicyclo[3.3.1]nonane, 0.5M in THF 99%View Details
280-64-8 -
![9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8](https://img.chemicalbook.in//Content/image/CP5.jpg) 9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8View Details
9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8View Details
280-64-8 -
![9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8](https://img.chemicalbook.in//Content/image/CP5.jpg) 9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8View Details
9-Borabicyclo[3.3.1]nonane solution CAS 280-64-8View Details
280-64-8 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
