N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
- CAS NO.:477600-73-0
- Empirical Formula: C20H25N5
- Molecular Weight: 335.45
- MDL number: MFCD16877475
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 14:23:51
![N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine Structural](https://img.chemicalbook.in/CAS/GIF/477600-73-0.gif)
What is N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine?
The Uses of N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine is an important pharmaceutical intermediate used for the synthesis of tofacitinib. Tofacitinib is a Janus kinase (JAK) inhibitor drug used to treat rheumatoid arthritis and psoriatic arthritis.
Synthesis
N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine is prepared by the reaction of 4-chloropyrrolopyrimidine and (3R,4R)-1-benzyl-N,4-dimethylpiperidin-3-amine dihydrochloride. The specific synthesis steps are as follows:
The reaction flask is added to sodium hydroxide (10.0g, 250mmol), water and dissolved, make 10% of the solution, adding acetone to another reaction bottle 80g, the toluene sulfonyl chloride (38.13g, 200mmol), after stirring to dissolve, then adding 4-chloro pyrrolo pyrimidine (15.36g, 100mmol), stirring mixing, cooling to 0 °C the following, dropping sodium hydroxide solution, the temperature is controlled at 5 °C the following, completion of the dropping, heating, to control the temperature to 20-30 °C stirring within the range of the reaction, to the reaction end after TLC monitoring, filtering, to get the product into the reaction bottle, adding water 200 ml, (3R, 4R)-cis-1-benzyl-4-methyl-3-methylamino-piperidine dihydrochloride (21.33g, 105mmol), stirring to dissolve, then adding potassium carbonate (82.93g, 600mmol), stirring, heating 95 °C, TLC monitoring to the reaction end of the, cooling to 45-55°C, by adding acetonitrile, preserving heat and stirring 1 hour, cooling to room temperature, crystallization, filtration, washing, the wet articles in added in the reaction bottle, adding dimethyl sulfoxide 250 ml, by adding 50% sodium hydroxide solution to 250 ml, stirring and heating 95 °C, TLC monitoring to the reaction end rear, layered, water extraction once with dimethyl sulfoxide, merger dimethyl asian sulphone level, cooling to 75-85°C, water slowly under stirring, the stirring cooling to room temperature, filtering, washing, 50% ethanol washing, filtering, drying, be [(3R, 4R) - 1 benzyl-4-methyl-piperidin-3-yl]-methyl-(7H-pyrrolo [2,3-d] pyrimidin-4-yl)-amine 25.83g, yield 77.0%, optical purity 99.8% (HPLC method).![N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine synthesis N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine synthesis](/NewsImg/2024-01-11/6384057056171848491778669.png)
Properties of N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
| Melting point: | 67-69℃ |
| Density | 1.205±0.06 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| pka | 13.36±0.50(Predicted) |
Safety information for N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
Computed Descriptors for N-((3R,4R)-1-benzyl-4-methylpiperidin-3-yl)-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine](https://img.chemicalbook.in/CAS/GIF/477600-74-1.gif)
![4-CHLORO-7-TOSYL-7H-PYRROLO[2,3-D]PYRIMIDINE](https://img.chemicalbook.in/CAS/GIF/479633-63-1.gif)
![7H-Pyrrolo[2,3-d]pyriMidin-4-aMine, N-Methyl-N-[(3R,4R)-4-Methyl-1-(phenylMethyl)-3-piperidinyl]-7-[(4-Met hylphenyl)sulfonyl]-](https://img.chemicalbook.in/CAS/20150408/GIF/923036-30-0.gif)
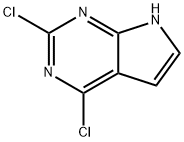
![4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](https://img.chemicalbook.in/CAS/GIF/3680-69-1.gif)
![3-PiperidinaMine, 1-acetyl-N,4-diMethyl-N-1H-pyrrolo[2,3-d]pyriMidin-4-yl-, (3R,4R)- (9CI)](https://img.chemicalbook.in/CAS/20150408/GIF/477600-76-3.gif)
![3-((3S,4R)-4-Methyl-3-(Methyl(7H-pyrrolo[2,3-d]pyriMidin-4-yl)aMino)piperidin-1-yl)-3-oxopropanenitrile](https://img.chemicalbook.in/CAS/GIF/1092578-48-7.gif)
You may like
-
![[(3R,4R)-1-benzyl-4-methyl-piperidin-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/6e57a743-4832-4f97-95bf-b142073c11b6.png) [(3R,4R)-1-benzyl-4-methyl-piperidin-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine 98%View Details
[(3R,4R)-1-benzyl-4-methyl-piperidin-3-yl]-methyl-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-amine 98%View Details -
![N-((3R,4R)-1-Benzyl-4-methylpiperidin-3-yl)-n-methyl-7h-pyrrolo[2,3-d]pyrimidin-4-amine 96% CAS 477600-73-0](https://img.chemicalbook.in//Content/image/CP5.jpg) N-((3R,4R)-1-Benzyl-4-methylpiperidin-3-yl)-n-methyl-7h-pyrrolo[2,3-d]pyrimidin-4-amine 96% CAS 477600-73-0View Details
N-((3R,4R)-1-Benzyl-4-methylpiperidin-3-yl)-n-methyl-7h-pyrrolo[2,3-d]pyrimidin-4-amine 96% CAS 477600-73-0View Details
477600-73-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
