5-ETHYL-2'-DEOXYURIDINE
Synonym(s):Edoxudine;EUdR
- CAS NO.:15176-29-1
- Empirical Formula: C11H16N2O5
- Molecular Weight: 256.26
- MDL number: MFCD00079191
- EINECS: 239-226-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
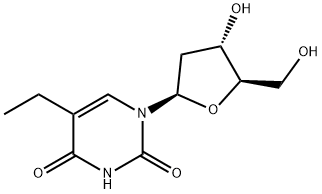
What is 5-ETHYL-2'-DEOXYURIDINE?
Absorption
Edoxudine cream is able to penetrate the skin in a very rapid manner. This easy penetration allows edoxudine to have a greater activity when compared with other topical antivirals that have better antiviral activity in vitro. In preclinical trials in mice, after intravenous administration of edoxudine, the mean residence time was 25 min. Edoxudine presented a bioavailability of 49% with a Cmax and tmax of 2.4 mcg/g and 31.1 min respectively. The AUC in plasma of edoxudine is significantly higher when administered orally when compared with intravenous administration.
Toxicity
Edoxudine is been reported to present cytotoxic effects and some degree of DNA incorporation in human leukemic cells and PHA-stimulated lymphocytes in vitro.
Chemical properties
Edoxudine is solid
The Uses of 5-ETHYL-2'-DEOXYURIDINE
Edoxudine was found to be a modulator of both antitumor action and pharmacokinetics of 5-Fluorouracil (F596000), a potent antineoplastic agent in clinical use.
The Uses of 5-ETHYL-2'-DEOXYURIDINE
5-Ethyl-2’-deoxyuridine was found to be a modulator of both antitumor action and pharmacokinetics of 5-Fluorouracil (F596000), a potent antineoplastic agent in clinical use.
The Uses of 5-ETHYL-2'-DEOXYURIDINE
antiallergic, mast cell degranulation inhibitor, angiogenesis blocker
Background
Edoxudine is a deoxythymidine analog with activity against herpes simplex virus. It is a potent and selective inhibitor of herpes simplex virus type 1 and 2. The obtained product is an antiviral ointment. The activity of edoxudine against herpes simplex virus was first recognized in 1967. It was later recognized to be effective in vivo in a preclinical model of keratitis caused by herpes virus. It was developed by McNeil Pharmaceutical and approved by Health Canada on December 31, 1992. This medication was later discontinued from the market in 1998.
What are the applications of Application
Edoxudine is a 5-fluorouracil (FU) modulator
Indications
Edoxudine was used in Europe, in the form of a topical antiviral, for the treatment of human herpes keratitis. Human herpes keratitis is an inflammation of the cornea in the eye caused by herpes simplex virus infection. This infection is a cause of significant morbidity whose incidence is significantly increased in the presence of recurrent infection and it can even produce corneal blindness.
Edoxudine 3% cream was also indicated for the treatment of dermal herpes simplex virus. This virus can produce an infection ubiquitously and it is highly contagious. There are two types of herpes virus, type 1 that is mainly transmitted by oral-to-oral contact and type 2 that is sexually transmitted.
Definition
ChEBI: Edoxudine is a pyrimidine 2'-deoxyribonucleoside.
Biochem/physiol Actions
5-Ethyl-2′-deoxyuridine (EUdR) is used as a 5-fluorouracil (FU) modulator. EtdUrd may be used to enhance the therapeutic index of 5-FU by reducing the catabolism, prolonging the plasma and intratumoral concentrations of 5-FU, and offering protection to normal organs by increasing the endogenous uridine levels.
Pharmacokinetics
In reports, it has been indicated that at antivirally active doses, edoxudine is phosphorylated to a much greater extent by hepatitis-infected cells when compared to mock-infected cells. Once phosphorylated, edoxudine is more highly incorporated into viral DNA than cellular DNA. The level of incorporation into viral DNA highly seems to be correlated with the concentration of edoxudine. The suppression of viral DNA synthesis caused a shutoff of viral replication and the viral titration is significantly reduced.The effect of edoxudine is also proven to reduce significantly the lesion area produced by the viral activity to an even 44% reduction.
Metabolism
In preclinical trials it has been reported that edoxudine presents a biotransformation marked by a cleavage of the glycoside bond. The degradation of edoxudine, after oral administration, seems to be processed by the activity of phosphorylases presented in the gastrointestinal tract and by pre-systemic metabolism.
Properties of 5-ETHYL-2'-DEOXYURIDINE
| Melting point: | 152-153° |
| Density | 1.389±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Sonicated), Water (Slightly) |
| form | Solid |
| pka | 9.98(at 25℃) |
| color | Pale Beige to Beige |
| Stability: | Hygroscopic |
Safety information for 5-ETHYL-2'-DEOXYURIDINE
Computed Descriptors for 5-ETHYL-2'-DEOXYURIDINE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



![5-[N-(N-BIOTINYL-EPSILON-AMINOCAPROYL)-3-AMINOALLYL]-2'-DEOXYURIDINE-5'-TRIPHOSPHATE](https://img.chemicalbook.in/StructureFile/ChemBookStructure1/GIF/CB3482348.gif)


You may like
-
 5-Ethyl-2′-deoxyuridine CAS 15176-29-1View Details
5-Ethyl-2′-deoxyuridine CAS 15176-29-1View Details
15176-29-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8