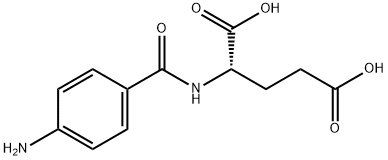
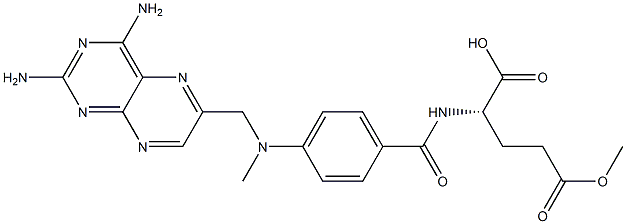
4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
- CAS NO.:19741-14-1
- Empirical Formula: C15H15N7O2
- Molecular Weight: 325.33
- MDL number: MFCD00075774
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
![4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID Structural](https://img.chemicalbook.in/CAS/GIF/19741-14-1.gif)
What is 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID?
Chemical properties
Brownish Yellow to Orange Solid
The Uses of 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
DAMPA is a Methotrexate (M260675) analog
The Uses of 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
DAMPA is a Methotrexate (M260675) analog (1). DAMPA is an antitumor agent (2).
Properties of 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
| Melting point: | 255 °C (dec.)(lit.) |
| Boiling point: | 689.3±65.0 °C(Predicted) |
| Density | 1.532 |
| storage temp. | 2-8°C(protect from light) |
| solubility | Aqueous Base, DMSO |
| form | neat |
| pka | 4.63±0.10(Predicted) |
| form | Solid |
| color | Dark Yellow to Very Dark Brown |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 19741-14-1 |
Safety information for 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-[N-(2,4-DIAMINO-6-PTERIDINYLMETHYL)-N-METHYLAMINO] BENZOIC ACID
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
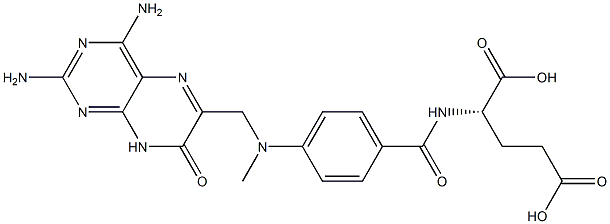
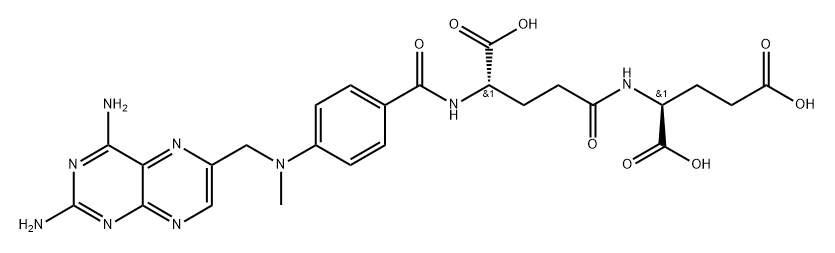

![L-Glutamic acid, N-[N-[N-[N-[4-[[(2, 4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-L-.gamma.-gluta myl]-L-.gamma.-glutamyl]-L-.gamma.-glutamyl]-](https://img.chemicalbook.in/CAS/20211123/GIF/73610-81-8.gif)

You may like
-
 Methotrexate impurity E CAS 19741-14-1View Details
Methotrexate impurity E CAS 19741-14-1View Details
19741-14-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.