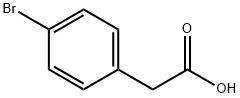
4-Bromobenzoic acid
- CAS NO.:586-76-5
- Empirical Formula: C7H5BrO2
- Molecular Weight: 201.02
- MDL number: MFCD00002529
- EINECS: 209-581-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:24
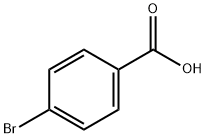
What is 4-Bromobenzoic acid?
Chemical properties
White to light yellow crystal powder
The Uses of 4-Bromobenzoic acid
4-Bromobenzoic acid was used to study the metabolic fate of 2-,3-and 4-bromo benzoic acids in rat hepatocytes incubation using high temperature liquid chromatography. It was used in bromine-specific detection of the metabolites of 2-,3-and 4-bromobenzoic acid in the urine and bile of rats by inductively coupled plasma mass spectrometry. It is also used as an intermediate for the synthesis of agrochemicals, pharmaceutical, chemical intermediates and liquid crystals.
Definition
ChEBI: A bromobenzoic acid carrying a single bromo subsituent at the 4-position.
Synthesis Reference(s)
Synthetic Communications, 17, p. 457, 1987 DOI: 10.1080/00397918708063924
Tetrahedron Letters, 18, p. 4631, 1977
General Description
Colorless to red crystals.
Reactivity Profile
Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 4-Bromobenzoic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
ACUTE/CHRONIC HAZARDS: Explodes above its melting point.
Purification Methods
Crystallise the acid from MeOH, or MeOH/water mixture, 90% EtOH and Et2O. The methyl ester has m 81o from Et2O or dilute MeOH. The anilide has m 197o (from EtOH). [Male & Thorp J Am Chem Soc 35 269 1913, Lamneck J Am Chem Soc 76 406 1954, Vandenbelt et al. Anal Chem 26 926 1954, Beilstein 9 IV 1017.]
Properties of 4-Bromobenzoic acid
| Melting point: | 252-254 °C (lit.) |
| Boiling point: | 116 °C(Press: 15-16 Torr) |
| Density | 1,894 g/cm3 |
| refractive index | 1.6080 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | ethanol: soluble5% |
| pka | 3.96(at 25℃) |
| form | Crystals or Powder |
| color | White to beige to pink-brownish |
| Water Solubility | SOLUBLE IN HOT WATER |
| Merck | 14,1408 |
| BRN | 1906923 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 586-76-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzoic acid, 4-bromo-(586-76-5) |
| EPA Substance Registry System | 4-Bromobenzoic acid (586-76-5) |
Safety information for 4-Bromobenzoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for 4-Bromobenzoic acid
| InChIKey | TUXYZHVUPGXXQG-UHFFFAOYSA-N |
4-Bromobenzoic acid manufacturer
JSK Chemicals
SAKEM LLP
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 586-76-5 4-Bromo benzoic acid 99%View Details
586-76-5 4-Bromo benzoic acid 99%View Details
586-76-5 -
 586-76-5 4-Bromo benzoic acid 98%View Details
586-76-5 4-Bromo benzoic acid 98%View Details
586-76-5 -
 586-76-5 4-Bromobenzoic acid 98%View Details
586-76-5 4-Bromobenzoic acid 98%View Details
586-76-5 -
 4-Bromobenzoic Acid CAS 586-76-5View Details
4-Bromobenzoic Acid CAS 586-76-5View Details
586-76-5 -
 4-Bromobenzoic acid CAS 586-76-5View Details
4-Bromobenzoic acid CAS 586-76-5View Details
586-76-5 -
 4-Bromobenzoic acid CAS 586-76-5View Details
4-Bromobenzoic acid CAS 586-76-5View Details
586-76-5 -
 4-Bromobenzoic acidView Details
4-Bromobenzoic acidView Details
586-76-5 -
 4-Bromo Benzoic AcidView Details
4-Bromo Benzoic AcidView Details
586-76-5
