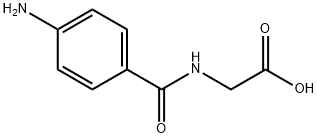
4-Aminohippuric acid
Synonym(s):N-(4-Aminobenzoyl)glycine;4-Aminohippuric acid;N-(4-Aminobenzoyl)aminoacetic acid, N-(4-Aminobenzoyl)glycine
- CAS NO.:61-78-9
- Empirical Formula: C9H10N2O3
- Molecular Weight: 194.19
- MDL number: MFCD00007890
- EINECS: 200-518-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
What is 4-Aminohippuric acid?
Toxicity
The intravenous LD50 in female mice is 7.22 g/kg.
Description
4-Aminohippuric acid is a substrate for various renal transporters that has been used in the study of anion uptake in the kidney. It is a substrate for organic anion transporter 1 (OAT1; Km = 14.3 μM in X. laevis oocytes), the human inorganic phosphate transporter (NPT1; Km = 2.66 mM in HEK293 cells), and apical multidrug resistance protein (MDR2; Km = 880 μM in HEK293 cells). 4-Aminohippuric acid is a glycine amide form of 4-aminobenzoic acid . Formulations containing 4-aminohippuric acid have been used as markers to determine renal plasma flow.
Chemical properties
off-white to greyish crystalline powder
The Uses of 4-Aminohippuric acid
4-Aminohippuric Acid is used in the measurement of renal plasma flow as a diagnostic tool which may be applied towards kidney disorders.
The Uses of 4-Aminohippuric acid
muscle relaxant (smooth)
The Uses of 4-Aminohippuric acid
p-Aminohippuric acid has been used as infusions to measure plasma flow.
Indications
Used to measure effective renal plasma flow (ERPF) and to determine the functional capacity of the tubular excretory mechanism.
Background
The glycine amide of 4-aminobenzoic acid. Its sodium salt is used as a diagnostic aid to measure effective renal plasma flow (ERPF) and excretory capacity. [PubChem]
Definition
ChEBI: P-aminohippuric acid is an N-acylglycine that is the 4-amino derivative of hippuric acid; used as a diagnostic agent in the measurement of renal plasma flow. It has a role as a Daphnia magna metabolite. It is a conjugate acid of a p-aminohippurate.
General Description
Renal tubules secretes para amino hippuric acid/PAH.
Biochem/physiol Actions
Para-aminohippuric acid(PAH) is used as a marker in nutrition studies to measure the flow of blood in pigs, which inturn helps to determine the portal-drained viscera (PDV) flux of nutrients.
Pharmacokinetics
Aminohippurate (p-aminohippuric acid, PAH, PAHA) is the glycine amide of p-aminobenzoic acid. It is filtered by the glomeruli and is actively secreted by the proximal tubules. At low plasma concentrations (1.0 to 2.0 mg/100 mL), an average of 90 percent of aminohippurate is cleared by the kidneys from the renal blood stream in a single circulation. It is ideally suited for measurement of ERPF since it has a high clearance, is essentially nontoxic at the plasma concentrations reached with recommended doses, and its analytical determination is relatively simple and accurate. Aminohippurate is also used to measure the functional capacity of the renal tubular secretory mechanism or transport maximum (TmPAH). This is accomplished by elevating the plasma concentration to levels (40-60 mg/100 mL) sufficient to saturate the maximal capacity of the tubular cells to secrete aminohippurate. Inulin clearance is generally measured during TmPAH determinations since glomerular filtration rate (GFR) must be known before calculations of secretory Tm measurements can be done.
Synthesis
4-Aminohippuric acid is prepared by reacting p-nitrobenzoyl chloride with glycine, followed by reducing the p-nitro group with tin and hydrochloric acid.
Metabolism
Not Available
Purification Methods
Crystallise the acid from H2O. It is soluble in organic solvents. [Cohen & McGilvery J Biol Chem 169 119 1945, 171 121 1947, Meunzen et al. J Biol Chem 26 469 1926, Beilstein 14 III 1069, 14 IV 1152.]
References
[1] B. ZMBOVA . Synthesis of p-aminohippuric acid analog and its labeling by technetium-99m[J]. International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes, 1989, 40 3: Pages 225-234. DOI:10.1016/0883-2889(89)90152-4.
[2] W P DEISS P P C. Studies in para-aminohippuric acid synthesis in the human: its application as a liver function test.[J]. Journal of Clinical Investigation, 1950, 29 8: 1014-1020. DOI:10.1172/JCI102332.
[3] M. SHEAT M. M Zenab F Saeed. Synthesis of Some of Azo, Triazines and Schiff Bases Compounds from 4-Aminohippuric Acid[J]. Journal of Education Science, 2009, 47 1: 1-8. DOI:10.33899/edusj.2009.57930.
[4] T SEKINE. Expression cloning and characterization of a novel multispecific organic anion transporter.[J]. The Journal of Biological Chemistry, 1997, 272 30: 18526-18529. DOI:10.1074/jbc.272.30.18526.
[5] HIROSHI UCHINO . p-Aminohippuric Acid Transport at Renal Apical Membrane Mediated by Human Inorganic Phosphate Transporter NPT1[J]. Biochemical and biophysical research communications, 2000, 270 1: Pages 254-259. DOI:10.1006/bbrc.2000.2407.
Properties of 4-Aminohippuric acid
| Melting point: | 199-200 °C (dec.)(lit.) |
| Boiling point: | 330.62°C (rough estimate) |
| Density | 1.356 |
| refractive index | 1.5600 (estimate) |
| storage temp. | Store below +30°C. |
| solubility | DMF: 33 mg/ml; DMF:PBS (pH7.2) (1:30): 0.03 mg/ml; DMSO: 20 mg/ml |
| pka | pKa 3.8 (Uncertain) |
| form | Crystalline Powder |
| color | Off-white to grayish |
| PH | 3.0-3.5 (20g/l, H2O, 20℃) |
| Water Solubility | Soluble in alcohol, chloroform, benzene, and acetone. Insoluble in water, ether, carbon, and tetrachloride. |
| Merck | 14,442 |
| BRN | 1213676 |
| CAS DataBase Reference | 61-78-9(CAS DataBase Reference) |
| EPA Substance Registry System | P-Aminohippuric acid (61-78-9) |
Safety information for 4-Aminohippuric acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Aminohippuric acid
| InChIKey | HSMNQINEKMPTIC-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


![diethyl N-[4-(methylamino)benzoyl]-L-glutamate](https://img.chemicalbook.in/CAS/GIF/2378-95-2.gif)
You may like
-
 61-78-9 4-Amino hippuric acid 99%View Details
61-78-9 4-Amino hippuric acid 99%View Details
61-78-9 -
 4-Aminohippuric Acid CAS 61-78-9View Details
4-Aminohippuric Acid CAS 61-78-9View Details
61-78-9 -
 Aminohippuric acid CAS 61-78-9View Details
Aminohippuric acid CAS 61-78-9View Details
61-78-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1