4-(2-FURYL)-3-BUTEN-2-ONE
- CAS NO.:623-15-4
- Empirical Formula: C8H8O2
- Molecular Weight: 136.15
- MDL number: MFCD00039566
- EINECS: 210-774-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
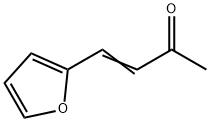
What is 4-(2-FURYL)-3-BUTEN-2-ONE?
Chemical properties
reddish crystalline powder
Chemical properties
4-(2-Furyl)-3-buten-2-one has a sweet spicy odor and taste It is useful in nut favors.
Occurrence
Reported found in coffee and rum
The Uses of 4-(2-FURYL)-3-BUTEN-2-ONE
4-(2-Furyl)-3-buten-2-one, can be used in technical or engineered material industyr. It has shown to have the durability need in composites.
Production Methods
4-(2-Furyl)-3-buten-2-one is produced by condensation of Furfural with Acetone.
Taste threshold values
Taste characteristics at 20 ppm: sweet, nutty, powdery, vanilla and coumarin creamy.
Synthesis Reference(s)
Journal of the American Chemical Society, 106, p. 6735, 1984 DOI: 10.1021/ja00334a044
The Journal of Organic Chemistry, 52, p. 4855, 1987 DOI: 10.1021/jo00231a006
General Description
Reddish crystalline solid.
Air & Water Reactions
Slightly water soluble.
Reactivity Profile
An aldehyde and a ketone. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Fire Hazard
4-(2-FURYL)-3-BUTEN-2-ONE is flammable.
Properties of 4-(2-FURYL)-3-BUTEN-2-ONE
| Melting point: | 34-41 °C(lit.) |
| Boiling point: | 112-115°C 10mm |
| Density | 1.0496 |
| refractive index | n |
| FEMA | 2495 | 4-(2-FURYL)-3-BUTEN-2-ONE |
| Flash point: | 220 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2–8 °C |
| solubility | Insoluble in water, soluble in alcohol and most oils |
| form | powder to lump |
| color | Light yellow to Brown |
| Odor | at 1.00 % in propylene glycol. sweet spicy warm balsam cinnamon vanilla |
| Sensitive | Light Sensitive |
| JECFA Number | 1511 |
| BRN | 109696 |
| Stability: | Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 623-15-4(CAS DataBase Reference) |
| EPA Substance Registry System | Furfural acetone (623-15-4) |
Safety information for 4-(2-FURYL)-3-BUTEN-2-ONE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 4-(2-FURYL)-3-BUTEN-2-ONE
4-(2-FURYL)-3-BUTEN-2-ONE manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![1-(4-METHOXYPHENYL)-3-[5-(4-NITROPHENYL)-2-FURYL]PROP-2-EN-1-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB1351521.gif)
![1-(4-METHYLPHENYL)-3-[5-(4-NITROPHENYL)-2-FURYL]PROP-2-EN-1-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB2739993.gif)

![4-(5-[3-(4-CHLOROPHENYL)-3-OXOPROP-1-ENYL]-2-FURYL)BENZENE-1-SULFONAMIDE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB5491191.gif)

You may like
-
 623-15-4 FURFURILIDINE ACETONE 98%View Details
623-15-4 FURFURILIDINE ACETONE 98%View Details
623-15-4 -
 623-15-4 99%View Details
623-15-4 99%View Details
623-15-4 -
 4-(2-Furyl)-3-buten-2-one CAS 623-15-4View Details
4-(2-Furyl)-3-buten-2-one CAS 623-15-4View Details
623-15-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1