3,5-Dimethoxytoluene
- CAS NO.:4179-19-5
- Empirical Formula: C9H12O2
- Molecular Weight: 152.19
- MDL number: MFCD00015435
- EINECS: 224-048-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
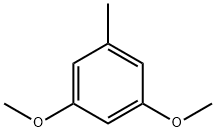
What is 3,5-Dimethoxytoluene?
Chemical properties
Clear colorless to yellow liquid
The Uses of 3,5-Dimethoxytoluene
3,5-Dimethoxytoluene (DMT) may be used in the synthesis of 3,5-dimethoxybenzoic acid by oxidation and 2-methoxy-6-methyl-1,4-benzoquinone by catalytic oxidation with hydrogen peroxide (H2O2)/methyltrioxorhenium (CH3ReO3) in dimethyl carbonate (DMC).
The Uses of 3,5-Dimethoxytoluene
3,5-Dimethoxytoluene has been suggested for use in perfume compositions as supporting note for Oakmoss and Vetiver, and as a novel note in Chypre, Fougere and Oriental fragrance types.
Definition
ChEBI: 3,5-dimethoxytoluene is a member of the class of toluenes that is toluene in which the hydrogens at positions 3 and 5 have been replaced by methoxy groups. It is the major scent compound of many rose varieties. It has a role as a fragrance and a plant metabolite. It is a member of toluenes and a member of methoxybenzenes.
Production Methods
3,5-Dimethoxytoluene can be produced from Orcinol in weak aqueous alkali with Dimethylsulfate at controlled temperature.
Synthesis Reference(s)
Organic Syntheses, Coll. Vol. 6, p. 859, 1988
The Journal of Organic Chemistry, 70, p. 3275, 2005 DOI: 10.1021/jo050075r
General Description
3,5-Dimethoxytoluene (DMT) is a methoxylated phenolic derivative. It is reported to be one of the main constituent of the floral volatiles in different rose varieties. It has been biosynthesized from orcinol by two successive methylation catalyzed by O-methyltransferases (OMTs). The features of its aerobic oxidation with metal/bromide catalysts have been investigated.
Properties of 3,5-Dimethoxytoluene
| Melting point: | 61-62 C |
| Boiling point: | 244 °C (lit.) |
| Density | 1.039 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 215 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Sparingly), Methanol (Sparingly) |
| form | Liquid |
| color | Clear colorless to yellow |
| Specific Gravity | 1.039 |
| Odor | Warm and sweet, nut-like, earthy-mossy odor |
| BRN | 2043558 |
| CAS DataBase Reference | 4179-19-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 3,5-Dimethoxytoluene(4179-19-5) |
| EPA Substance Registry System | 3,5-Dimethoxytoluene (4179-19-5) |
Safety information for 3,5-Dimethoxytoluene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3,5-Dimethoxytoluene
3,5-Dimethoxytoluene manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4179-19-5 5-Methylresorcinol dimethyl ether 98%View Details
4179-19-5 5-Methylresorcinol dimethyl ether 98%View Details
4179-19-5 -
 3,5-Dimethoxytoluene CAS 4179-19-5View Details
3,5-Dimethoxytoluene CAS 4179-19-5View Details
4179-19-5 -
 3,5-Dimethoxytoluene CAS 4179-19-5View Details
3,5-Dimethoxytoluene CAS 4179-19-5View Details
4179-19-5 -
 3,5-Dimethoxytoluene CAS 4179-19-5View Details
3,5-Dimethoxytoluene CAS 4179-19-5View Details
4179-19-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
