3-METHYLPENTANE
- CAS NO.:96-14-0
- Empirical Formula: C6H14
- Molecular Weight: 86.18
- MDL number: MFCD00009342
- EINECS: 202-481-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
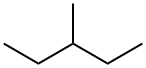
What is 3-METHYLPENTANE?
Chemical properties
colourless liquid
Chemical properties
3-Methylpentane, C6H14, is a colorless, flammable liquid with specific gravity 0.664. It occurs in petroleum and natural gas, and may be released to the environment in evaporative losses, wastewater, spills, and combustion exhaust.
Physical properties
Clear, colorless, flammable liquid with an odor similar to hexane, heptane, and similar aliphatic hydrocarbons. An odor threshold concentration of 8.9 ppmv was reported by Nagata and Takeuchi (1990).
The Uses of 3-METHYLPENTANE
3-Methylpentane is a component of three typical commercial hexanes, obtained from the fractionation of natural gas liquids, a refinery operation involving hydrogenation, and a stream meeting polymerization. Other than for fuel, it is used in extraction of oil from seeds and as a solvent and reaction medium in the manufacture of polyolefins, synthetic rubbers, and some pharmaceuticals.
The Uses of 3-METHYLPENTANE
Organic synthesis, solvent.
The Uses of 3-METHYLPENTANE
3-Methylpentane is used as a solvent in the preparation of vegetable oils, glues, coatings, and paints. It is also used in gasoline, rubber solvent, and petroleum ether.
What are the applications of Application
3-Methylpentane is A simple alkyl compound
Definition
ChEBI: 3-methylpentane is an alkane that is pentane which is substituted by a methyl group at position 3. It is used as a solvent in organic synthesis, as a lubricant and as a raw material for producing carbon black. It has a role as a human metabolite, an allelochemical and a non-polar solvent. It is an alkane and a volatile organic compound.
Hazard
Flammable, dangerous fire risk.
Safety Profile
May have narcotic or anesthetic properties. A very dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Source
Schauer et al. (1999) reported 3-methylpentane in a diesel-powered medium-duty truck
exhaust at an emission rate of 670 μg/km.
California Phase II reformulated gasoline contained 3-methylpentane at a concentration of 22.7
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 3.76 and 512 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Photolytic. The following rate constants were reported for the reaction of 3-methylpentane and
OH radicals in the atmosphere: 4.30 x 10-9 cm3/molecule?sec at 300 K (Darnall et al., 1976); 6.8 x
10-12 cm3/molecule?sec at 305 K (Darnall et al., 1978); 5.7 x 10-12 cm3/molecule?sec (Altshuller,
1991).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor. 3-
Methylpentane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Purify it by azeotropic distillation with MeOH, as for 2-methylpentane. Purify it for ultraviolet spectroscopy by passing it through columns of silica gel or alumina activated by heating for 8hours at 210o under a stream of nitrogen. Alternatively treat it with conc (or fuming) H2SO4, then wash it with water, aqueous 5% NaOH, water again, then dry (CaCl2, then sodium), and distil it through a long, glass helices-packed, column. [Beilstein 1 IV 363.]
Properties of 3-METHYLPENTANE
| Melting point: | -118 °C |
| Boiling point: | 64 °C(lit.) |
| Density | 0.664 g/mL at 25 °C(lit.) |
| vapor density | 2.97 (vs air) |
| vapor pressure | 135 mm Hg ( 17 °C) |
| refractive index | n |
| Flash point: | 20 °F |
| storage temp. | Flammables area |
| solubility | In methanol, g/L: 389 at 5 °C, 450 at 10 °C, 530 at 15 °C, 650 at 20 °C, 910 at 25 °C. Miscible at
higher temperatures (Kiser et al., 1961). |
| form | Liquid |
| color | Clear colorless |
| explosive limit | ~7.7% |
| Odor Threshold | 8.9ppm |
| Water Solubility | Soluble in water. 0.013 g/L at 20°C). Miscible in ether, acetone. Soluble in ethanol. |
| BRN | 1730734 |
| Henry's Law Constant | 1.693 atm?m3/mol at 25 °C (Hine and Mookerjee, 1975) |
| Exposure limits | ACGIH TLV: TWA and STEL for all isomers except n-hexane are 500 and
1,000 ppm, respectively (adopted). |
| Dielectric constant | 1.8899999999999999 |
| Stability: | Stable. Highly flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 96-14-0(CAS DataBase Reference) |
| EPA Substance Registry System | 3-Methylpentane (96-14-0) |
Safety information for 3-METHYLPENTANE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-METHYLPENTANE
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![1,2,3,4,5,6-HEXAMETHYLBICYCLO[2.2.0]HEXA-2,5-DIENE](https://img.chemicalbook.in/CAS/GIF/7641-77-2.gif)
You may like
-
 3-Methylpentane CAS 96-14-0View Details
3-Methylpentane CAS 96-14-0View Details
96-14-0 -
 3-Methylpentane CAS 96-14-0View Details
3-Methylpentane CAS 96-14-0View Details
96-14-0 -
 3-Methylpentane CAS 96-14-0View Details
3-Methylpentane CAS 96-14-0View Details
96-14-0 -
 3-Methylpentane CAS 96-14-0View Details
3-Methylpentane CAS 96-14-0View Details
96-14-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1