3-METHYLHEXANE
- CAS NO.:589-34-4
- Empirical Formula: C7H16
- Molecular Weight: 100.2
- MDL number: MFCD00009408
- EINECS: 209-643-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
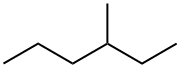
What is 3-METHYLHEXANE?
Chemical properties
CLEAR COLOURLESS LIQUID
Physical properties
Clear, colorless liquid with an odor similar to hexane. An odor threshold concentration of 840 ppbv was reported by Nagata and Takeuchi (1990).
The Uses of 3-METHYLHEXANE
3-Methylhexane can be used as a model alkane to study:
- Cu-catalyzed hydrocarboxylation of simple alkanes to corresponding branched monocarboxylic acids in aqueous medium at low-temperature.
- Au-catalyzed oxygenation of alkanes with H2O2 in acetonitrile.
- FeCI3 catalyzed photooxygenation of simple alkanes to corresponding alcohols in presence of atmospheric O2.
The Uses of 3-METHYLHEXANE
Organic synthesis, oil extender solvent.
Definition
ChEBI: 3-methylhexane is an alkane that is hexane substituted by a methyl group at position 3. It has a role as a human metabolite. It is an alkane and a volatile organic compound.
Hazard
Flammable, dangerous fire risk.
Source
Schauer et al. (1999) reported 3-methylhexane in a diesel-powered medium-duty truck
exhaust at an emission rate of 310 μg/km.
California Phase II reformulated gasoline contained 3-methylhexane at a concentration of 16.2
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 2.95 and 415 mg/km, respectively (Schauer et al., 2002).
Environmental Fate
Photolytic. Based on a reported photooxidation reaction rate constant of 7.20 x 10-12
cm3/molecule?sec with OH radicals, the half-life of 3-methylhexane is 20 h (Altshuller, 1990).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water vapor. 3-
Methylhexane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Purify it as for 2-methylhexene. [Beilstein 1 IV 399.]
Properties of 3-METHYLHEXANE
| Melting point: | −119 °C(lit.) |
| Boiling point: | 91 °C(lit.) |
| Density | 0.687 g/mL at 25 °C(lit.) |
| vapor pressure | 2.13 psi ( 37.7 °C) |
| refractive index | n |
| Flash point: | 25 °F |
| solubility | Soluble in acetone, alcohol, benzene, chloroform, ligroin, and ether (Weast, 1986). Miscible with
liquid aliphatic hydrocarbons. |
| form | clear liquid |
| color | Colorless to Almost colorless |
| Odor Threshold | 0.84ppm |
| explosive limit | ~7% |
| Water Solubility | 2.64mg/L(25 ºC) |
| BRN | 1718739 |
| Henry's Law Constant | 2.38 at 25 °C (Mackay and Shiu, 1981) |
| Dielectric constant | 1.9299999999999999 |
| CAS DataBase Reference | 589-34-4(CAS DataBase Reference) |
| EPA Substance Registry System | 3-Methylhexane (589-34-4) |
Safety information for 3-METHYLHEXANE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P273:Avoid release to the environment. P331:Do NOT induce vomiting. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for 3-METHYLHEXANE
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran
You may like
-
 3-Methylhexane CAS 589-34-4View Details
3-Methylhexane CAS 589-34-4View Details
589-34-4 -
 3-Methylhexane CAS 589-34-4View Details
3-Methylhexane CAS 589-34-4View Details
589-34-4 -
 Ethoxymethylenemalononitrile 99% HPLCView Details
Ethoxymethylenemalononitrile 99% HPLCView Details
123-06-8 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 401-95-6 99% HPLCView Details
401-95-6 99% HPLCView Details
401-95-6 -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1