3-Dimethylaminophenol
Synonym(s):N,N-Dimethyl-3-aminophenol
- CAS NO.:99-07-0
- Empirical Formula: C8H11NO
- Molecular Weight: 137.18
- MDL number: MFCD00002264
- EINECS: 202-727-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-28 09:12:15
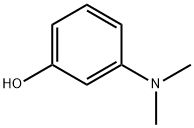
What is 3-Dimethylaminophenol?
Chemical properties
Brown to Purple Solid
The Uses of 3-Dimethylaminophenol
3-Dimethylaminophenol can be used as neostigmine bromide intermediate and dye intermediate.
General Description
Needles (from ligroin) or a dark gray solid.
Air & Water Reactions
Sensitive to prolonged exposure to air. Insoluble in water.
Reactivity Profile
An amine and phenol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid.
Fire Hazard
Flash point data for 3-Dimethylaminophenol are not available. 3-Dimethylaminophenol is probably combustible.
Properties of 3-Dimethylaminophenol
| Melting point: | 82-84 °C(lit.) |
| Boiling point: | 265-268°C |
| Density | 1.0976 (rough estimate) |
| refractive index | 1.5895 (estimate) |
| Flash point: | 148 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 10.06±0.10(Predicted) |
| form | Powder |
| color | violet |
| PH | 7 (10g/l, H2O, 20℃)(slurry) |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Light Sensitive |
| BRN | 774631 |
| Stability: | Stable. May be air-sensitive. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 99-07-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 3-(dimethylamino)-(99-07-0) |
| EPA Substance Registry System | m-(Dimethylamino)phenol (99-07-0) |
Safety information for 3-Dimethylaminophenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Dimethylaminophenol
| InChIKey | MESJRHHDBDCQTH-UHFFFAOYSA-N |
3-Dimethylaminophenol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 99-07-0 3-(Dimethylamino)phenol 98%View Details
99-07-0 3-(Dimethylamino)phenol 98%View Details
99-07-0 -
 99-07-0 98%View Details
99-07-0 98%View Details
99-07-0 -
 3-Dimethyl amino phenol 97-07-0 98%View Details
3-Dimethyl amino phenol 97-07-0 98%View Details
97-07-0 -
 99-07-0 3-(dimethylamino)phenol 99%View Details
99-07-0 3-(dimethylamino)phenol 99%View Details
99-07-0 -
 3-Dimethylaminophenol, 97% CAS 99-07-0View Details
3-Dimethylaminophenol, 97% CAS 99-07-0View Details
99-07-0 -
 3-(Dimethylamino)phenol CAS 99-07-0View Details
3-(Dimethylamino)phenol CAS 99-07-0View Details
99-07-0 -
 3-(Dimethylamino)phenol CAS 99-07-0View Details
3-(Dimethylamino)phenol CAS 99-07-0View Details
99-07-0 -
 3-(dimethyl amino)phenol CAS NO: 99-07-0View Details
3-(dimethyl amino)phenol CAS NO: 99-07-0View Details
99-07-0
