2(5H)-Furanone
Synonym(s):γ-Crotonolactone;2-Buten-1,4-olide
- CAS NO.:497-23-4
- Empirical Formula: C4H4O2
- Molecular Weight: 84.07
- MDL number: MFCD00005376
- EINECS: 207-839-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-23 18:28:04
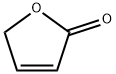
What is 2(5H)-Furanone?
Chemical properties
2(5H)-Furanone is light brown liquid
Chemical properties
Colorless to pale brown-yellow clear liquid
Occurrence
Reported found in coffee
The Uses of 2(5H)-Furanone
2(5H)-Furanone is a versatile reagent used in Michael addition reactions1 for synthesis of lignans.2 Also employed in three-component Michael-Aldol reactions with an aldehyde anda thiolate3 or carbanion.4
The Uses of 2(5H)-Furanone
2(5H)-Furanone is a heterocyclic organic compound that is used widely in the synthetic preparation of pharmaceutical goods.
The Uses of 2(5H)-Furanone
2(5H)-Furanone is employed in the preparation of 5-substituted 2(5H) furanones (gamma-butenolides) catalyzed by bifunctional aminothiourea and aminosquaramide organocatalysts through direct aldol reaction with aromatic aldehydes. Further, it is used in the synthesis of lignans by Michael addition reactions. In addition to this, it is used in three-component Michael-Aldol reactions with an aldehyde and thiolate or carbanion.
Definition
2(5H)-Furanone is a mycotoxin that contains an α,β-unsaturated lactone moiety.
General Description
Chiral urea compounds catalyzed hetero-Michael addition reaction of 2(5H)-furanone (γ-crotonolactone) to pyrrolidine. The quorum sensing inhibition activity by 2(5H)-furanone was studied using bioindicator strains.
Hazard
Extremely toxic.
Purification Methods
Fractionally distil the lactone under reduced pressure. IR: (CCl4) 1784 and 1742 cm-1, UV no max above 205nm ( 1160 cm-1 M-1) and 1HR: (CCl3) : 2.15 (pair of triplets 1H), 3.85 (pair of triplets 1H) and 5.03 (triplet 2H). [Price & Judge Org Synth Coll Vol V 255 1973, Smith & Jones Can J Chem 37 2007, 2092 1959, Beilstein 17/9 V 112.]
Properties of 2(5H)-Furanone
| Melting point: | 4-5 °C (lit.) |
| Boiling point: | 86-87 °C/12 mmHg (lit.) |
| Density | 1.185 g/mL at 25 °C (lit.) |
| refractive index | n |
| FEMA | 4138 | 4-HYDROXY-2-BUTENOIC ACID GAMMA-LACTONE |
| Flash point: | 214 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform, Ethyl Acetate (Slightly) |
| form | Liquid |
| color | Clear colorless to pale yellow or amber |
| Odor | buttery |
| Water Solubility | Immiscible with water. |
| JECFA Number | 2000 |
| BRN | 383585 |
| Stability: | Moisture Sensitive |
| CAS DataBase Reference | 497-23-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2(5H)-Furanone(497-23-4) |
| EPA Substance Registry System | 2(5H)-Furanone (497-23-4) |
Safety information for 2(5H)-Furanone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 2(5H)-Furanone
| InChIKey | VIHAEDVKXSOUAT-UHFFFAOYSA-N |
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 2-(5H)- Furanone 98%View Details
2-(5H)- Furanone 98%View Details -
 γ-Crotonolactone CAS 497-23-4View Details
γ-Crotonolactone CAS 497-23-4View Details
497-23-4 -
 2(5H)-Furanone, 98% CAS 497-23-4View Details
2(5H)-Furanone, 98% CAS 497-23-4View Details
497-23-4 -
 2(5H)-Furanone CAS 497-23-4View Details
2(5H)-Furanone CAS 497-23-4View Details
497-23-4 -
 2(5H)-Furanone CAS 497-23-4View Details
2(5H)-Furanone CAS 497-23-4View Details
497-23-4 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4