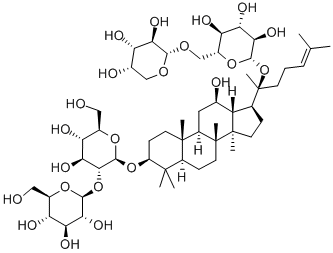
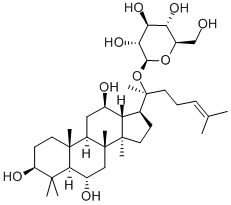
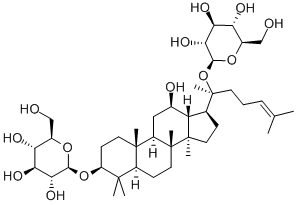
20(R)-Ginsenoside Rg3
- CAS NO.:38243-03-7
- Empirical Formula: C42H72O13
- Molecular Weight: 785.01
- MDL number: MFCD11045225
- EINECS: 233-305-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
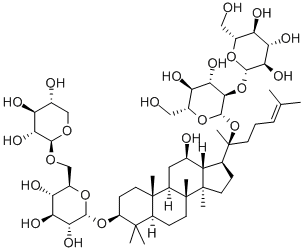
What is 20(R)-Ginsenoside Rg3?
Description
Ginsenoside Rg3 is mainly derived from the dried roots of Araliaceae Panax ginseng C.A.?Mey. and Panax quinquefolius L.?Ginseng has a long history of medicinal uses, well known as “king of herbs.” Ginseng is listed as the top grade in “Shen Nong’s herbal class,” with “the main security set up the five internal organs, spirit, soul, stop fright, in addition to evil, eyesight, fun puzzle” account. The traditional Chinese medicine regards ginseng as the top grade of reinforcing Qi, with Dabu strength, spleen and lungs, Sheng Jin, Anshen Yizhi, etc.
Physical properties
Appearance: white powder. Density: 1.40. Melting point: 315–318°C. Solubility: 20(R)-ginsenoside Rg3 is soluble in methanol and ethanol; low water solubility; insoluble in ether and chloroform. Storage temperature: 2–8°C.The component classification belongs to three dammarane tetracyclic triterpenoid saponins.
History
Ginseng is currently one of the most studied traditional medicines. Chemical researches certificate that ginseng contains a variety of ginseng saponin, ginseng polysaccharide, protein, polypeptide, amino acids, and other chemical constituents. Ginseng saponins are the main active components, accounting for about 4%. Ginsenoside is a kind of sterol compound, which has a similar basic structure. Ginsenoside contains the steroid nucleus of 17 carbon atoms arranged in 4 rings. At present, more than 40 kinds of ginsenosides have been isolated from ginseng. They are divided into four types according to their chemical structure, the protopanaxadiol, the protopanaxatriol, the ocotillol, and the oleanolic acid.
Ginsenoside Rg3 is a dammarane tetracyclic triterpenoid saponin found in natural ginseng, which belongs to the protopanaxadiol . It is also the natural ingredient with prominent activity extracted from red ginseng (processed ginseng). Modern
medicine has proved that Rg3 has certain biological activity. Since 1983, Rg3 has
been widely studied by many scholars in the world . The results showed that the
content of ginsenoside Rg3 in the fresh and dried ginseng roots was less than 1 ppm
while in red ginseng was 3/100,000. After several years of research, the method of
extracting 20(R)-ginsenoside Rg3 from red ginseng has been successfully improved,
and the industrialized production has been realized. A multicenter clinical study by
eight domestic hospitals indicated that 20(R)-ginsenoside Rg3 has therapeutic
effects on tumor. It has been issued the new drug certificate by the Chinese Food and
Drug Administration, as a new anticancer drug approved.
The Uses of 20(R)-Ginsenoside Rg3
20(R)-Ginsenoside Rg3 causes an inhibition of phosphatidylinositol 3-kinase/Akt activation that, in turn, results in modulations in Bcl-2 family proteins in such a way that the apoptosis of U87 cells are regulated. Elicits a significant inhibition of in vitro cell adhesion and invasion of U87 cells. in vitro stereoselective inhibition of ginsenosides toward UDP-glucuronosyltransferase isoforms and in vivo inhibition of tumor angiogenesis of lung cancer.
Indications
This product is contained in “New drug standards” the 87th volume.
Ginsenoside Rg3 capsule, ginsenoside Rg3 emulsion, and ginsenoside Rg3 microemulsion are mainly used in the clinical treatment or adjuvant therapy of lung cancer, breast cancer, liver cancer, gastric cancer, colon cancer, etc. In addition, they improve and prevent the common diseases in the elderly such as cardiovascular disease, coronary heart disease, limb weakness, difficulty walking, and loss of memory. Ginsenoside Rg3 also has a certain biological activity in antiviral, radiosensitization, and treatment of chronic obstructive pulmonary disease.
Definition
Ginsenoside Rg3 is a tetracyclic triterpenoid saponin type rich in red ginseng. The major ginsenosides, such as Rb1, Rb2, and Rg3. Due to the different spatial structures from C20 positions, there are two enantiomers, 20(R) and 20(S)-isomers. With differential conguration, their antitumor activities exhibit certain differences. 20(R)-ginsenoside Rg3 and 20(S)-ginsenoside Rg3 can inhibit tumor metastasis by the suppression of EMT. 20(R)-ginsenoside Rg3 suppressed lung cancer migration or/and invasion by inhib-iting TGF-β1-induced EMT accompanied by the inactivation of MMP-2, p38 MAPK and Smad2. In addition, 20(S)-ginsenoside Rg3 effectively suppressed hypoxia-induced EMT, inhibiting ovarian cancer metastasis[1].
Pharmacology
It is reported that the antitumor effects of ginsenoside Rg3 and Rh2 were the most significant in more than 40 kinds of ginsenosides obtained. Moreover, they enhanced the immune function of tumor patients after chemotherapy, including humoral immunity, cell immunity, and non-specific immunity . This effect is consistent with the vitality nourishing and body strengthening of ginseng in the theory of traditional Chinese medicine. There is more research on the effects of 20(R)-ginsenoside Rg3?in the two isomers of ginsenoside Rg3, which has certain inhibitory effects on tumor cells of lung cancer, gastric cancer, colon cancer, breast cancer, liver cancer, etc. There is less research on the effects of 20(S)-ginsenoside Rg3. Other studies have shown that ginsenoside Rg3 also has the effects of anti-fatigue , antiviral , and treatment of chronic obstructive pulmonary disease .
Human tolerance test shows that ginsenoside Rg3 has a wide safety range. The
recommended dose of the first phase of clinical trial (0.8 mg/kg/day) has no side
effects. Ginsenoside Rg3 has good oral absorption. The drug absorption peak in the
blood is detected after administration for 15–30 min, and the blood concentration
reaches the peak after administration for 1.0–1.5 h. The half-life is 4.84±1.41 h. The maximum blood concentration increases with the dose within the reagent range
(0.8–3.2 mg/kd/day), suggesting the pharmacokinetics of ginsenoside Rg3 the firstorder kinetics process.
Clinical Use
The basic research for many years and a large number of clinical data confirm that ginsenoside Rg3 effectively inhibits the growth of lung cancer, liver cancer, gastric cancer, colon cancer, and breast cancer, significantly improves the clinical symptoms of patients, improves the quality of life, and prevents and treats cancer. Ginsenoside Rg3 effectively improves the ability of anti-fatigue, antiaging, and resistance to disease. It also improves cardiovascular function, is resistant to platelet aggregation, protects nerve cells of brain, and improves immunity.
Ginsenoside Rg3 is the active ingredient of Shenyi capsule which is a
kind of traditional Chinese anticancer medicine developed in China. It prevents the
metastasis and recurrence of cancer and has been an adjuvant treatment in multiple
tumors . Shenyi capsule improves the curative effects of primary lung cancer and
liver cancer, relieves the symptoms of Qi deficiency, as well as improves immunity,
when combined with chemotherapy. Further study on the antitumor mechanism of
ginsenoside Rg3 will be helpful to the clinical application of this drug.
References
[1] Central Laboratory. “Anticancer effects of ginsenoside Rg3 (Review).”International Journal of Molecular Medicine 39 3 (2017): 507-518.
Properties of 20(R)-Ginsenoside Rg3
| Melting point: | 315-318 ºC |
| Boiling point: | 885.0±65.0 °C(Predicted) |
| Density | 1.30 |
| storage temp. | Hygroscopic, -20°C Freezer, Under inert atmosphere |
| solubility | Chloroform (Very Slightly, Heated Sonicated), Pyridine (Slightly, Heated, Sonica |
| form | Solid |
| pka | 12.85±0.70(Predicted) |
| color | White to Off-White |
| Stability: | Hygroscopic |
Safety information for 20(R)-Ginsenoside Rg3
Computed Descriptors for 20(R)-Ginsenoside Rg3
| InChIKey | VMFHKVMQSYOBAV-KQVQOVSDNA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 Ginsenoside rb3 95% CAS 38243-03-7View Details
Ginsenoside rb3 95% CAS 38243-03-7View Details
38243-03-7 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
