2-methylpentane
Synonym(s):‘Isohexane’;2-Methylpentane;iso-Hexane, 1-Dimethylbutane
- CAS NO.:107-83-5
- Empirical Formula: C6H14
- Molecular Weight: 86.18
- MDL number: MFCD00009406
- EINECS: 203-523-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
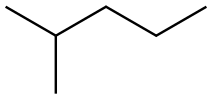
What is 2-methylpentane?
Chemical properties
colourless liquid
Chemical properties
2-Methylpentane (isohexane), C6H14, is a flammable liquid with a specific gravity of 0.653. It occurs naturally in petroleum and gas and as a plant volatile. It is found in sources associated with petroleum products such as petroleum manufacture, natural gas, turbines, and automobiles.
Physical properties
Clear, colorless, very flammable liquid with an odor similar to hexane. An odor threshold concentration of 8.9 ppmv was reported by Nagata and Takeuchi (1990).
The Uses of 2-methylpentane
2-Methylpentane is employed as a raw material, rubber solvent and vegetable oil extraction solvent. It is also used as a solvent in adhesives. Further, it is used as an intermediate in organic synthesis and finds application in food, preservatives, cosmetics, pharmaceuticals, beverages and flavor enhancer.
The Uses of 2-methylpentane
Organic synthesis, solvent.
The Uses of 2-methylpentane
2-Methylpentane is mainly used in studies involving the functionalization of aliphatic C–H bonds using different carbene insertion processes to form C–H insertion products. The metal-free Ritter-type amination reaction of tertiary C–H bond using iodic acid as an oxidant in the presence of N-hydroxyphthalimide has also been reported.
Production Methods
Isohexane is manufactured by fractional distillation of gasoline derived from crude oil or liquid product derived from natural gas.
Definition
ChEBI: 2-Methylpentane is an alkane.
General Description
Watery liquid with a gasoline-like odor, Floats on water. Produces an irritating vapor.
Air & Water Reactions
Highly flammable.
Reactivity Profile
Saturated aliphatic hydrocarbons, such as ISOHEXENE, may be incompatible with strong oxidizing agents like nitric acid. Charring of the hydrocarbon may occur followed by ignition of unreacted hydrocarbon and other nearby combustibles. In other settings, aliphatic saturated hydrocarbons are mostly unreactive. They are not affected by aqueous solutions of acids, alkalis, most oxidizing agents, and most reducing agents.
Hazard
Flammable, dangerous fire risk, reacts vig-orously with oxidizing materials.
Health Hazard
Inhalation causes irritation of respiratory tract, cough, mild depression, cardiac arrhythmias. Aspiration causes severe lung irritation, coughing, pulmonary edema; excitement followed by depression. Ingestion causes nausea, vomiting, swelling of abdomen, headache, depression.
Source
Schauer et al. (1999) reported 2-methylpentane in a diesel-powered medium-duty truck
exhaust at an emission rate of 930 μg/km.
A constituent in gasoline. Harley et al. (2000) analyzed the headspace vapors of three grades of
unleaded gasoline where ethanol was added to replace methyl tert-butyl ether. The gasoline vapor
concentrations of 2-methylpentane in the headspace were 9.3 wt % for regular grade, 9.8 wt % for mid-grade, and 10.4 wt % for premium grade.
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of 2-methylpentane was 8.6 mg/kg of pine burned. Emission rates of 2-methylpentane were
not measured during the combustion of oak and eucalyptus.
California Phase II reformulated gasoline contained 2-methylpentane at a concentration of 36.9
g/kg. Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 6.31 and 827 mg/km, respectively (Schauer et al., 2002).
Reported as an impurity (0.1 wt %) in 99.0–99.7 wt % 2,3-dimethylbutane (Chevron Phillips,
2004).
Environmental Fate
Photolytic. When synthetic air containing gaseous nitrous acid and 2-methylpentane was
exposed to artificial sunlight (λ = 300–450 nm), acetone, propionaldehyde, peroxyacetal nitrate,
peroxypropionyl nitrate, and possibly two isomers of hexyl nitrate and propyl nitrate formed as
products (Cox et al., 1980).
Based on a photooxidation rate constant of 5.6 x 10-12 cm3/molecule?sec for the reaction of 2-
methylpentane and OH radicals, the atmospheric lifetime is 25 h (Altshuller, 1991).
Chemical/Physical: Complete combustion in air yields carbon dioxide and water vapor. 2-
Methylpentane will not hydrolyze because it does not contain a hydrolyzable functional group.
Purification Methods
Purify it by azeotropic distillation with MeOH, followed by washing out the MeOH with water, drying (CaCl2, then sodium), and distilling it. [Forziati et al. J Res Nat Bur Stand 36 129 1946.]
Properties of 2-methylpentane
| Melting point: | -154 °C (lit.) |
| Boiling point: | 62 °C (lit.) |
| Density | 0.653 g/mL at 25 °C (lit.) |
| vapor density | 3 (vs air) |
| vapor pressure | 6.77 psi ( 37.7 °C) |
| refractive index | n |
| Flash point: | −10 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.14g/l |
| form | Liquid |
| color | Clear colorless |
| explosive limit | 1.2-7.4%(V) |
| Odor Threshold | 7ppm |
| Water Solubility | Miscible with alcohol, ether, acetone and chloroform. Immiscible with water. |
| BRN | 1730735 |
| Henry's Law Constant | 0.697, 0.694, 0.633, 0.825, and 0.848 (atm?m3/mol)at 10, 15, 20, 25, and 30 °C, respectively (EPICS, Ashworth
et al., 1988) |
| Exposure limits | ACGIH TLV: TWA and STEL for all isomers except n-hexane are 500 and
1,000 ppm, respectively (adopted). |
| Dielectric constant | 1.8899999999999999 |
| Stability: | Stable. Highly flammable. Gas/vapour mixtures explosive at some concentrations. |
| CAS DataBase Reference | 107-83-5(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Methylpentane (107-83-5) |
Safety information for 2-methylpentane
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H336:Specific target organ toxicity,single exposure; Narcotic effects H373:Specific target organ toxicity, repeated exposure H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 2-methylpentane
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2-Methylpentane CAS 107-83-5View Details
2-Methylpentane CAS 107-83-5View Details
107-83-5 -
 2-Methylpentane CAS 107-83-5View Details
2-Methylpentane CAS 107-83-5View Details
107-83-5 -
 Hexane, mixture of isomers CAS 107-83-5View Details
Hexane, mixture of isomers CAS 107-83-5View Details
107-83-5 -
 Hexane, mixture of isomers CAS 107-83-5View Details
Hexane, mixture of isomers CAS 107-83-5View Details
107-83-5 -
 2-Methylpentane CAS 107-83-5View Details
2-Methylpentane CAS 107-83-5View Details
107-83-5 -
 2-Methylpentane CAS 107-83-5View Details
2-Methylpentane CAS 107-83-5View Details
107-83-5 -
 Hexane, mixture of isomers CAS 107-83-5View Details
Hexane, mixture of isomers CAS 107-83-5View Details
107-83-5 -
 2-Methylpentane CAS 107-83-5View Details
2-Methylpentane CAS 107-83-5View Details
107-83-5