2-ETHYLBUTYRALDEHYDE
Synonym(s):2-Ethylbutanal
- CAS NO.:97-96-1
- Empirical Formula: C6H12O
- Molecular Weight: 100.16
- MDL number: MFCD00006985
- EINECS: 202-623-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-18 17:01:59
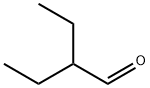
What is 2-ETHYLBUTYRALDEHYDE?
Description
Ethyl butyraldehyde is a colorless liquid.Molecular weight = 100.18; Boiling point = 116.8℃;Freezing/Melting point = - 89℃; Flash point = 21℃ (oc).Explosive limits: LEL = 1.2%; UEL = 7.7%. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 3, Reactivity 1. Insoluble in water.
Chemical properties
Colorless liquid.Insoluble in water.
Chemical properties
Ethyl butyraldehyde is a colorless liquid
Chemical properties
2-Ethylbutyraldehyde has a pungent odor.
Occurrence
Reported found in melon, French fried potato, wheaten bread, scallops, citrus fruits, white bread and maize.
The Uses of 2-ETHYLBUTYRALDEHYDE
2-Ethylbutyraldehyde has been used in the preparation of aldoximes using aqueous hydroxylamine.
The Uses of 2-ETHYLBUTYRALDEHYDE
Organic synthesis, pharmaceuticals, rubber accelerators, synthetic resins.
Definition
ChEBI: 2-Ethylbutanal is an organooxygen compound.
Taste threshold values
Taste characteristics at 20 ppm: green, fruity, cocoa with sweet, fresh nuances.
General Description
A clear colorless liquid. Flash point 70°F. Less dense than water and insoluble in water. Vapors heavier than air.
Air & Water Reactions
Highly flammable. With air slowly form peroxides. Insoluble in water.
Reactivity Profile
2-ETHYLBUTYRALDEHYDE is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.
Hazard
Irritant to eyes and skin. Flammable, dangerous fire risk.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by ingestion. Mildly toxic by inhalation. A skin irritant. Flammable liquid. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, Co2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.
Synthesis
From diethyl carbinol and anhydrous oxalic acid or with sulfuric acid; a more recent synthetic route (Xeisel–Neuwirth method) calls for the reduction of α-vinylcrotonaldehyde using iron dust and acetic acid
Potential Exposure
Used in organic synthesis of pharmaceuticals and rubber chemicals.
First aid
this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. Store intightly closed containers in a cool, well-ventilated areaaway from oxidizers. Where possible, automatically pumpliquid from drums or other storage containers to processcontainers
Shipping
UN1178 2-Ethyl butyraldehyde, Hazard Class: 3; Labels: 3-Flammable liquid
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and reducing agents. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of 2-ETHYLBUTYRALDEHYDE
| Melting point: | -89°C |
| Boiling point: | 117 °C(lit.) |
| Density | 0.814 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 2426 | 2-ETHYLBUTYRALDEHYDE |
| Flash point: | 70 °F |
| storage temp. | Flammables area |
| form | clear liquid |
| color | Colorless to Light yellow |
| Odor | at 10.00 % in dipropylene glycol. sweet green ethereal fruity cocoa |
| Water Solubility | Soluble in ether and alcohols. Slightly soluble in water. |
| Sensitive | Air Sensitive |
| JECFA Number | 256 |
| BRN | 1209330 |
| Stability: | Stability Stable, but air sensitive. Flammable. Readily forms explosive mixtures with air. Incompatible with strong bases, strong reducing agents, oxidizing agents. |
| CAS DataBase Reference | 97-96-1(CAS DataBase Reference) |
| EPA Substance Registry System | Butanal, 2-ethyl- (97-96-1) |
Safety information for 2-ETHYLBUTYRALDEHYDE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-ETHYLBUTYRALDEHYDE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 2-Ethylbutyraldehyde CAS 97-96-1View Details
2-Ethylbutyraldehyde CAS 97-96-1View Details
97-96-1 -
 2-Ethylbutyraldehyde CAS 97-96-1View Details
2-Ethylbutyraldehyde CAS 97-96-1View Details
97-96-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4