2'-Deoxyguanosine monohydrate
- CAS NO.:312693-72-4
- Empirical Formula: C10H15N5O5
- Molecular Weight: 285.26
- MDL number: MFCD00150760
- EINECS: 628-392-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
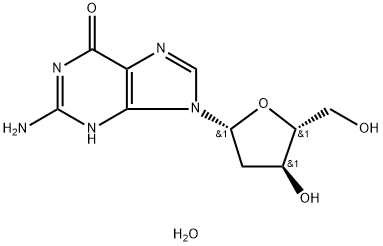
What is 2'-Deoxyguanosine monohydrate?
Description
2''-Deoxyguanosine is a purine nucleoside with diverse biological activities. It inhibits the clonogenic growth of HL-60 and K562 leukemia cells (IC50s = 80 and 100 μM, respectively). 2''-Deoxyguanosine inhibits the growth of MOLT-4 T cells and MGL-8 B cells by 99.8 and 68.3%, respectively, when used at a concentration of 50 μM. It increases the number of binucleated cells, a marker of inhibited cytokinesis, in A. sativum meristems. 2''-Deoxyguanosine (>1 μM) also induces relaxation of precontracted isolated bovine lingual artery.
Chemical properties
White to Off-White Solid. Soluble in NH4OH 1 M: 50 mg/mL, clear to very faintly turbid.
The Uses of 2'-Deoxyguanosine monohydrate
2′-Deoxyguanosine monohydrate has been used in stromal cell purification. It has also been used for the quantification of whole cell dNTP (deoxyribonucleotide triphosphate).
Definition
ChEBI: 2'-Deoxyguanosine monohydrate is a purine 2'-deoxyribonucleoside having guanine as the nucleobase. It has a role as a Saccharomyces cerevisiae metabolite, a human metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a purines 2'-deoxy-D-ribonucleoside and a purine 2'-deoxyribonucleoside. It is functionally related to a guanosine.
What are the applications of Application
2′-Deoxyguanosine monohydrate has been used as a nucleoside supplement:
to study its effect on mitochondrial DNA copy number in deoxyguanosine kinase (dguok) mutant zebrafish,
in tissue culture medium for deoxyribonucleotide triphosphate (dNTP) synthesis,
in RPMI (Roswell Park Memorial Institute)-1640 medium to eliminate the endogenous thymocytes.
Biological Functions
lu et al. found that 2'-Deoxyguanosine (2-dG) is a key immune signaling molecule in plant–microbe interactions and that Arabidopsis VENOSA4 (VEN4) is essential for plant immunity. 2-dG is the immunoreactive component in ZhiNengCong (ZNC). This compound induced classical immune responses, including ROS production, allose accumulation and MPKs phosphorylation, and upregulated the expression of plant immunity-associated genes. In addition, 2-dG-induced plant immunity requires salicylic acid (SA) receptor NPR1 (but not SA), ethylene (ET) signaling, pattern-recognition receptors/coreceptors (PRRs/coreceptors) and the ATP receptor P2K1[1]. VEN4 was involved in 2-dG biosynthesis and could convert dGTP to 2-dG.
Biochem/physiol Actions
Deoxyguanosine (dG) is a purine nucleoside that upon sequential phosphorylation (kinases) forms deoxyguanosine triphosphate (dGTP) which is used by DNA polymerases and reverse transcriptases to synthesize DNA(s). Deoxyguanosine is the most electron-rich of the four canonical bases and includes many nucleophilic sites which are susceptible to oxidative damage. This makes deoxyguanosine and its oxidized derivatives useful reagents to study mechanisms of oxidative damage to nucleosides and nucleotides.
References
[1] Chongchong Lu . “Discovery of a novel nucleoside immune signaling molecule 2′-deoxyguanosine in microbes and plants.” Journal of Advanced Research 46 (2023): Pages 1-15.
Properties of 2'-Deoxyguanosine monohydrate
| Melting point: | >300 °C (dec.)(lit.) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | NH4OH 1 M: 50 mg/mL, clear to very faintly turbid, yellow to brown |
| form | powder |
| pka | pK1:2.5(+1) (25°C,μ=0.1) |
| color | White to Off-White |
| BRN | 39814 |
| InChI | InChI=1/C10H13N5O4/c11-10-13-8-7(9(18)14-10)12-3-15(8)6-1-4(17)5(2-16)19-6/h3-6,16-17H,1-2H2,(H3,11,13,14,18)/t4-,5+,6+/s3 |
Safety information for 2'-Deoxyguanosine monohydrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P270:Do not eat, drink or smoke when using this product. P330:Rinse mouth. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P403:Store in a well-ventilated place. |
Computed Descriptors for 2'-Deoxyguanosine monohydrate
| InChIKey | LZSCQUCOIRGCEJ-FPKZOZHISA-N |
| SMILES | OC[C@H]1O[C@@H](N2C3=C(C(NC(=N3)N)=O)N=C2)C[C@@H]1O |&1:2,4,17,r| |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran


You may like
-
 2′-Deoxyguanosine monohydrate CAS 312693-72-4View Details
2′-Deoxyguanosine monohydrate CAS 312693-72-4View Details
312693-72-4 -
 2′-Deoxyguanosine monohydrate CAS 312693-72-4View Details
2′-Deoxyguanosine monohydrate CAS 312693-72-4View Details
312693-72-4 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
