2-BUTENE
- CAS NO.:624-64-6
- Empirical Formula: C4H8
- Molecular Weight: 56.11
- MDL number: MFCD00064458
- EINECS: 210-855-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30

What is 2-BUTENE?
Chemical properties
colourless gas
Chemical properties
2-Butene, C4H8, can occur in trans or cis conformation; the former is the more stable form. It is a colorless, extremely flammable gas. 2-Butene occurs in coal gas and has been detected in diesel exhaust. The more highly reactive trans-2-butene occurs at much lower concentrations in the atmosphere than other comparable hydrocarbons.
The Uses of 2-BUTENE
trans-2-Butene can be used as a monomer unit to produce high molecular weight polyolefins with well-defined microstructures by nickle-catalyzed polymerization reaction. It is also used as a reactant in the isomerization reaction to produce the corresponding isomer using acid-activated catalysts.
Production Methods
2-Butene has been recovered from refining gases or produced by petroleum cracking. It is a component in the production of gasolines, butadiene, and a variety of other chemicals.
Definition
ChEBI: But-2-ene is a butene.
General Description
A colorless liquefied petroleum gas. An asphyxiant. Flammability limits of 1.8-9% by volume.
Air & Water Reactions
Highly flammable. Insoluble in water.
Reactivity Profile
The unsaturated aliphatic hydrocarbons, such as 2-BUTENE, are generally much more reactive than the alkanes. Strong oxidizers may react vigorously with them. Reducing agents can react exothermically to release gaseous hydrogen. In the presence of various catalysts (such as acids) or initiators, compounds in this class can undergo very exothermic addition polymerization reactions. Aluminum borohydride reacts with alkenes and in the presence of oxygen, combustion is initiated even in the absence of moisture.
Purification Methods
The gas is dried with CaH2 and purified by gas chromatography. [Beilstein 1 H 205, 1 II 176, 1 III 730, 1 IV 781.] HIGHLY FLAMMABLE.
Properties of 2-BUTENE
| Melting point: | −140 °C(lit.) |
| Boiling point: | 1 °C(lit.) |
| Density | 0.5990 |
| vapor density | 2 (vs air) |
| vapor pressure | 2575 mm Hg ( 37.7 °C) |
| refractive index | 1.3932 |
| Flash point: | <−30 °F |
| explosive limit | 9.7% |
| Merck | 1520 |
| BRN | 1718756 |
| Stability: | Stable. Incompatible with strong oxidizing agents, peroxides, peroxyacids, aluminium tetrahydroborate. Extremely flammable. |
| CAS DataBase Reference | 624-64-6(CAS DataBase Reference) |
| EPA Substance Registry System | trans-2-Butene (624-64-6) |
Safety information for 2-BUTENE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H220:Flammable gases H280:Gases under pressure |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P410+P403:Protect from sunlight. Store in a well-ventilated place. |
Computed Descriptors for 2-BUTENE
| InChIKey | IAQRGUVFOMOMEM-ONEGZZNKSA-N |
2-BUTENE manufacturer
Vadilal Chemicals Limited
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
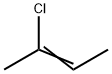
 624-64-6 trans-2-Butene 98%View Details
624-64-6 trans-2-Butene 98%View Details
624-64-6 -
 trans-2-Butene CAS 624-64-6View Details
trans-2-Butene CAS 624-64-6View Details
624-64-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8