1H,1H,2H,2H-Perfluorooctanesulfonic acid
- CAS NO.:27619-97-2
- Empirical Formula: C8H5F13O3S
- Molecular Weight: 428.17
- MDL number: MFCD00042455
- EINECS: 248-580-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
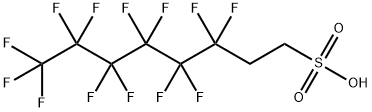
What is 1H,1H,2H,2H-Perfluorooctanesulfonic acid?
Description
1H,1H,2H,2H-Perfluorooctanesulfonic acid (1H,1H,2H,2H-Perfluorooctanesulphonic acid) is an efficient fluorine-containing surfactant without perfluorooctane sulfonic acid, which has the characteristics of high temperature resistance, oxidant resistance, electrolytic stability, etc. It can reduce the concentration of chromic acid in plating workshop and inhibit the precipitation of chromic acid and chromium mist.
Chemical properties
White to Brown Solid.
The Uses of 1H,1H,2H,2H-Perfluorooctanesulfonic acid
1H,1H,2H,2H-Perfluorooctanesulfonic acid is an aliphatic compound for fluorinated surfactant synthesis.
What are the applications of Application
3,3,4,4,5,5,6,6,7,7,8,8,8-Tridecafluorooctane-1-sulphonic acid is an aliphatic compound for fluorinated surfactant synthesis
What are the applications of Application
1H,1H,2H,2H-Perfluorooctanesulfonic acid (1H,1H,2H,2H-Perfluorooctanesulphonic acid) can functionalize gallium nitride (GaN) to tune the optical properties, which can potentially be used in chemical sensor based applications. It can modify the surface characteristics of copper substrates that find usage in printed circuit boards as copper foils. It can also be coated on the indium tin oxide substrate, which may be utilized in organic light emitting diodes (OLEDs) and organic photovoltaics (OPVs).
General Description
1H,1H,2H,2H-Perfluorooctanesulfonic acid is a fluoroalkyl phosphonic acid that contains 8 fluorinated carbon atoms. It forms a self-assembled layer on the substrate due to the adhesion of the phosphonates. The surface adhesion is a component of the acid base linkage that is enhanced by the hydroxyl groups.
Flammability and Explosibility
Not classified
Toxicology
Toxicological studies of 1H,1H,2H,2H-Perfluorooctanesulfonic acid (6:2 FTS) induced cardiovascular abnormalities in zebrafish embryos and larvae showed that the acute toxicity of 6:2 FTS was lower than that of PFOS. 6:2 FTS could affect the development of zebrafish, including increasing the utilization of yolk sac and the length of the pharyngeal pouch, leading to pericardial edema and inhibiting beating of the heart. Then transgenic zebrafish CZ63 and CZ40 were used to further explore its cardiovascular toxicity. 6:2 FTS could induce vascular hyperplasia, inhibit atrial development, and reduce blood flow velocity in larvae at 72 hours postfertilization. In addition, 6:2 FTS enhanced CAT and GSH-Px activities, involved in calcium signalling and myocardial contraction, and affected MAPK, FoxO and p53 signalling pathways[3].
Storage
2-8°C
Environmental considerations
Regulatory efforts are underway to limit the use of some Fluorinated additive material, mainly perfluorinated compounds (PFCs), due to concerns over their environmental persistency, bioaccumulation ability, and toxicity. Among the PFCs, perfluorosulfonates (PFSAs) and perfluorocarboxylates (PFCAs) are most widely detected in human and other biological samples, including those in pristine areas such as the Arctic. The longer-chained PFCs are known to be bioaccumulative and have toxic effects on biota. For example, neuroendocrine effects and peroxisome proliferation have been shown to occur. The perfluorinated sulfonamides are neutral compounds and are consequently not as water-soluble as the acids and are more volatile. It has been suggested that they are transformed into perfluorinated sulfonates (PFSAs) and PFCAs in the atmosphere. Since the perfluorinated acids have high water solubilities and low pKa values, they are dissociated at environmentally relevant pH values. They are primarily found in water or bound to particles, sediments, and soils[1-2].
References
[1] L. Ahrens. "Determination of polyfluoroalkyl compounds in water and suspended particulate matter in the river Elbe and North Sea, Germany." Frontiers of Environmental Science & Engineering in China 3.1(2009): 152–170.
[2] RuanTing. "Trace determination of airborne polyfluorinated iodine alkanes using multisorbent thermal desorption/gas chromatography/high resolution mass spectrometry." Journal of Chromatography A 1217 26 (2010): 4439–47.
[3] LING WANG, YAWEI WANG; Early Stage Exposure of 1H,1H,2H,2H-Perfluorooctanesulfonate-Induced Cardiovascular Abnormality in Zebrafish Embryos and Larvae[J]. ACS ES&T water, 2022. DOI:10.1021/acsestwater.2c00435.
Properties of 1H,1H,2H,2H-Perfluorooctanesulfonic acid
| Density | 1,25 g/cm3 |
| vapor pressure | 1.96Pa at 20℃ |
| solubility | Acetone: Slightly soluble,Chloroform: Slightly soluble,DMSO: Slightly soluble,Methanol: Slightly soluble |
| pka | 1.31±0.50(Predicted) |
| form | A low-melting solid |
| Water Solubility | 658g/L at 20℃ |
| InChI | InChI=1S/C8H5F13O3S/c9-3(10,1-2-25(22,23)24)4(11,12)5(13,14)6(15,16)7(17,18)8(19,20)21/h1-2H2,(H,22,23,24) |
| EPA Substance Registry System | 1-Octanesulfonic acid, 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluoro- (27619-97-2) |
Safety information for 1H,1H,2H,2H-Perfluorooctanesulfonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 1H,1H,2H,2H-Perfluorooctanesulfonic acid
| InChIKey | VIONGDJUYAYOPU-UHFFFAOYSA-N |
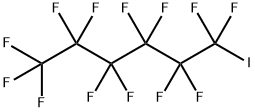
| SMILES | C(S(O)(=O)=O)CC(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)C(F)(F)F |
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran
You may like
-
 1H,1H,2H,2H-Perfluorooctanesulfonic acid CAS 27619-97-2View Details
1H,1H,2H,2H-Perfluorooctanesulfonic acid CAS 27619-97-2View Details
27619-97-2 -
 Sodium Stearyl Fumarate 98%View Details
Sodium Stearyl Fumarate 98%View Details
4070-80-8 -
 Testosterone Enanthate 315-37-7 98%View Details
Testosterone Enanthate 315-37-7 98%View Details
315-37-7 -
 121-54-0 Benzethonium Chloride 98%View Details
121-54-0 Benzethonium Chloride 98%View Details
121-54-0 -
 5721-91-5 98%View Details
5721-91-5 98%View Details
5721-91-5 -
 Calcium 11% clear solution 98%View Details
Calcium 11% clear solution 98%View Details -
 Sodium Croscarmellose 98%View Details
Sodium Croscarmellose 98%View Details
74811-65-7 -
 16455-61-1 98%View Details
16455-61-1 98%View Details
16455-61-1
