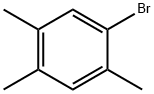
1,2,4-Trimethylbenzene
Synonym(s):1,2,4-Trimethylbenzene;Pseudocumene;Pseudocumol
- CAS NO.:95-63-6
- Empirical Formula: C9H12
- Molecular Weight: 120.19
- MDL number: MFCD00008527
- EINECS: 202-436-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:18
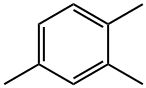
What is 1,2,4-Trimethylbenzene?
Description
1,2,4-Trimethylbenzene (mesitylene) is an aromatic hydrocarbon compound commonly present in commercial solvents encompassing their boiling range. Mesitylene are the most common isomer used in commercial applications. Aromatic hydrocarbons in hydrocarbon solvents are predominantly alkylated one ring structures as well as two aromatic ring structures which may also be alkylated.
Chemical properties
colourless liquid. Insoluble in water, soluble in ethanol, ether and benzene.
Physical properties
Colorless liquid with a slight aromatic odor. A detection odor threshold concentration of 12 mg/m3 (2.4 ppmv) was experimentally determined by Dravnieks (1974).
The Uses of 1,2,4-Trimethylbenzene
1,2,4-Trimethylbenzene is the unlabelled version of 1,2,4-Trimethyl 13C6-benzene (T796173), a labelled aromatic standard.
The Uses of 1,2,4-Trimethylbenzene
Sterilizing catgut by heating one hour at 160°; solvent in manufacture of dyes, perfumes and resins. Solvent for liquid scintillation counting solutions.
Definition
ChEBI: 1,2,4-trimethylbenzene is a trimethylbenzene carrying methyl groups at positions 1, 2 and 4. It has a role as a neurotoxin.
Synthesis Reference(s)
Journal of the American Chemical Society, 73, p. 766, 1951 DOI: 10.1021/ja01146a079
Chemical and Pharmaceutical Bulletin, 34, p. 540, 1986 DOI: 10.1248/cpb.34.540
General Description
A liquid. Flash point near 130°F. Less dense than water and insoluble in water. Vapors irritate eyes, throat, and nose. Used in dyes and pharmaceuticals.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,2,4-Trimethylbenzene is incompatible with the following: Oxidizers, nitric acid .
Hazard
Moderate fire risk. Central nervous system depressant, irritant to mucous membranes. Asthma and hematologic effects.
Health Hazard
Harmful if inhaled or swallowed. Vapor or mist is irritating to the eyes, mucous membrane and upper respiratory tract. Prolonged contact can cause dermatitis, nausea, headache, dizziness, and narcotic effect.
Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by inhalation. Can cause central nervous system depression, anemia, bronchitis. Flammable liquid when exposed to heat, sparks, or flame. To fight fire, use foam, alcohol foam, mist. Emitted from modern building materials (CENEAR 69,22,91). When heated to decomposition it emits acrid smoke and irritating fumes.
Toxicology
Acute toxicity studies (oral, dermal and inhalation routes of exposure) have been conducted in rats. Oral LD50 values of greater than 5000 mg/kg have been reported. For solvent products containing predominantly mixed C9 aromatic hydrocarbons, oral LD50 values have been reported as greater than 6880 mg/kg (males) and greater than 3440 mg/kg (females). LC50 values of 18,000 mg/m3 was reported for 1,2,4-TMB. For C9 aromatic solvents, the LC50 was greater than 10,200 mg/m3 (Shell Research Laboratory, 1976, unpublished study). One dermal acute study showed an LD50 value of greater than 3440 mg/kg for a C9 aromatic solvent (Shellsol A, a highly aromatic solvent boiling in the white spirit range, consisting of primarily C9 isomers particularly TMBs)
Environmental Fate
Biological. In anoxic groundwater near Bemidji, MI, 1,2,4-trimethylbenzene anaerobically
biodegraded to the intermediate 3,4-dimethylbenzoic acid and the tentatively identified
compounds 2,4- and/or 2,5-dimethylbenzoic acid (Cozzarelli et al., 1990).
Photolytic. Glyoxal, methylglyoxal, and biacetyl were produced from the photooxidation of
1,2,4-trimethylbenzene by OH radicals in air at 25 °C (Tuazon et al., 1986a). A rate constant of
2.0 x 10-8 L/molecule?sec was reported for the reaction of 1,2,4-trimethylbenzene with OH radicals
in the gas phase (Darnall et al., 1976). Similarly, the rate constants for the reaction of 1,2,4-
trimethylbenzene and OH radicals at room temperature were 3.35 x 10-11 (Hansen et al., 1975) and
3.84 x 10-11 cm3/molecule?sec (Atkinson, 1985). At 25 °C, a rate constant of 3.15 x 10-11
cm3/molecule?sec was reported for the same reaction (Ohta and Ohyama, 1985). Products
identified from the OH radical-initiated reaction of 1,2,4-trimethylbenzene in the presence of
nitrogen dioxide were 3-hexene-2,5-dione and 2,3-butanedione (Bethel et al., 2000).
Chemical/Physical. 1,2,4-Trimethylbenzene will not hydrolyze in water (Kollig, 1993).
Purification Methods
Reflux pseudocumene over sodium and distil it under reduced pressure. [Beilstein 6 H 1088, 6 I 542, 6 II 1072, 6 III 6278, 6 IV 7339.]
Properties of 1,2,4-Trimethylbenzene
| Melting point: | -44 °C |
| Boiling point: | 168 °C(lit.) |
| Density | 0.876 g/mL at 20 °C(lit.) |
| vapor density | 4.1 (vs air) |
| vapor pressure | 4.5 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | 120 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.057g/l |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Clear colorless |
| Odor | char. aromatic odor |
| Odor Threshold | 0.12ppm |
| explosive limit | 0.8-7%(V) |
| Water Solubility | Soluble in alcohol, benzene and ether. Slightly soluble in water |
| Merck | 14,7915 |
| BRN | 1903005 |
| Henry's Law Constant | 6.946, 11.202, and 15.702 at 27.0, 35.0, and 45.0 °C, respectively (dynamic headspace, Hansen et al., 1995) |
| Exposure limits | NIOSH REL: TWA 25 ppm (125 mg/m3); ACGIH TLV: TWA for mixed
isomers 25 ppm (adopted). |
| Dielectric constant | 2.4(16℃) |
| Stability: | Stable. Incompatible with strong oxidizing agents. Flammable. May form explosive mixtures with air. |
| CAS DataBase Reference | 95-63-6(CAS DataBase Reference) |
| EPA Substance Registry System | 1,2,4-Trimethylbenzene (95-63-6) |
Safety information for 1,2,4-Trimethylbenzene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2,4-Trimethylbenzene
| InChIKey | GWHJZXXIDMPWGX-UHFFFAOYSA-N |
1,2,4-Trimethylbenzene manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1 2 4-trimethylbenzeneView Details
1 2 4-trimethylbenzeneView Details
95-63-6 -
 1,2,4-Trimethylbenzene CAS 95-63-6View Details
1,2,4-Trimethylbenzene CAS 95-63-6View Details
95-63-6 -
 1 2 4 TRIMETHYLBENZENEView Details
1 2 4 TRIMETHYLBENZENEView Details
95-63-6
