1,2,3-Trimethylbenzene
Synonym(s):Hemellitol
- CAS NO.:526-73-8
- Empirical Formula: C9H12
- Molecular Weight: 120.19
- MDL number: MFCD00008520
- EINECS: 208-394-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
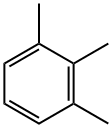
What is 1,2,3-Trimethylbenzene?
Chemical properties
colourless liquid
Chemical properties
Trimethylbenzenes exists in three isomeric forms. All isomers are clear, colorless liquids with a distinctive, aromatic odor.
Physical properties
Clear, colorless, flammable liquid with an aromatic odor similar to propylbenzene, ethylbenzenes, or xylenes.
The Uses of 1,2,3-Trimethylbenzene
Raw material for chemical synthesis.
Definition
ChEBI: A trimethylbenzene carrying methyl groups at positions 1, 2 and 3. It has been found in Centaurium erythraea.
Synthesis Reference(s)
Journal of the American Chemical Society, 62, p. 2639, 1940 DOI: 10.1021/ja01867a017
Organic Syntheses, Coll. Vol. 4, p. 508, 1963
Hazard
Combustible. Central nervous system impairment, asthma, and hematologic effects.
Safety Profile
Mildly toxic by ingestion, Flammable liquid when exposed to heat, sparks, or flame. To fight fire, use water spray, mist, dry chemical, CO2, foam. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
(1,2,3-and 1,2,4-isomers): These materials are used as solvents and in dye and perfume manufacture. The 1,2,3-isomer is used as raw material in chemical synthesis and as an UV stabilizer. The 1,2,4-isomer is used as the raw material for trimellitic anhydride manufacture. These compounds are found in diesel engine exhaust fumes.
Source
Detected in distilled water-soluble fractions of 87 octane gasoline (0.30 mg/L), 94 octane
gasoline (0.81 mg/L), Gasohol (0.80 mg/L), No. 2 fuel oil (0.22 mg/L), diesel fuel (0.09 mg/L),
and military jet fuel JP-4 (0.19 mg/L) (Potter, 1996).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
602. Average 1,2,3-trimethylbenzene concentrations detected in water-soluble fractions of
unleaded gasoline, kerosene, and diesel fuel were 1.219, 0.405, and 0.118 mg/L, respectively.
When the authors analyzed the aqueous-phase via U.S. EPA approved test method 610, average
1,2,3-trimethylbenzene concentrations in water-soluble fractions of unleaded gasoline, kerosene,
and diesel fuel were smaller, i.e., 742, 291, and 105 μg/L, respectively.
Environmental Fate
Photolytic. Glyoxal, methylglyoxal, and biacetyl were produced from the photooxidation of
1,2,3-trimethylbenzene by OH radicals in air at 25 °C (Tuazon et al., 1986a). The rate constant for
the reaction of 1,2,3-trimethylbenzene and OH radicals at room temperature was 1.53 x 10-11
cm3/molecule?sec (Hansen et al., 1975). A rate constant of 1.49 x 10-8 L/molecule?sec was reported
for the reaction of 1,2,3-trimethylbenzene with OH radicals in the gas phase (Darnall et al., 1976).
Similarly, a room temperature rate constant of 3.16 x 10-11 cm3/molecule?sec was reported for the
vapor-phase reaction of 1,2,3-trimethylbenzene with OH radicals (Atkinson, 1985). At 25 °C, a
rate constant of 2.69 x 10-11 cm3/molecule?sec was reported for the same reaction (Ohta and
Ohyama, 1985). 2,3-Butanedione was the only products identified from the OH radical-initiated
reaction of 1,2,4-trimethylbenzene in the presence of nitrogen dioxide. The amount of 2,3-
butanedione formed decreased with increased concentration of nitrogen dioxide (Bethel et al.,
2000).
Chemical/Physical. 1,2,3-Trimethylbenzene will not hydrolyze because it does not contain a
hydrolyzable group (Kollig, 1993).
Shipping
UN3295 Hydrocarbons, liquid, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid. UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required. 1,3,5-Trimethylbenzene; UN2325, Hazard Class: 3; Labels: 3-Flammable liquid. 1,2,4-Trimethylbenzene:
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of 1,2,3-Trimethylbenzene
| Melting point: | −25 °C(lit.) |
| Boiling point: | 175-176 °C(lit.) |
| Density | 0.894 g/mL at 25 °C(lit.) |
| vapor density | 4.15 (vs air) |
| vapor pressure | 3.4 mm Hg ( 37.7 °C) |
| refractive index | n |
| Flash point: | 119 °F |
| solubility | Soluble in acetone, alcohol, benzene, and ether (Weast, 1986) |
| pka | >14 (Schwarzenbach et al., 1993) |
| form | neat |
| color | Colorless to Light yellow |
| Odor Threshold | 0.032ppm |
| Water Solubility | 65.51mg/L(25 ºC) |
| BRN | 1903410 |
| Henry's Law Constant | 3.18 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 25 ppm (125 mg/m3); ACGIH TLV: TWA for mixed
isomers 25 ppm (adopted). |
| Dielectric constant | 2.6600000000000001 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 526-73-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2,3-trimethyl-(526-73-8) |
| EPA Substance Registry System | 1,2,3-Trimethylbenzene (526-73-8) |
Safety information for 1,2,3-Trimethylbenzene
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1,2,3-Trimethylbenzene
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 1,2,3-Trimethylbenzene CAS 526-73-8View Details
1,2,3-Trimethylbenzene CAS 526-73-8View Details
526-73-8 -
 1,2,3-Trimethylbenzene CAS 526-73-8View Details
1,2,3-Trimethylbenzene CAS 526-73-8View Details
526-73-8 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
