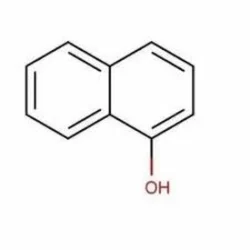
1-Naphthol
Synonym(s):α-Naphthol;1-Hydroxynaphthalene;1-Naphthol;Duloxetine impurity D (PhEur)
- CAS NO.:90-15-3
- Empirical Formula: C10H8O
- Molecular Weight: 144.17
- MDL number: MFCD00003930
- EINECS: 201-969-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:16
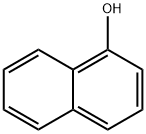
What is 1-Naphthol?
Description
1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula C10H7OH. It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol, with the hydroxyl group being more reactive than in the phenols. Both isomers are soluble in simple alcohols, ethers, and chloroform. They are precursors to a variety of useful compounds. Naphthols (both 1 and 2 isomers) are used as biomarkers for livestock and humans exposed to polycyclic aromatic hydrocarbons.
α-naphtol, combined with epichlorhydrine and sodium hydroxide to form alpha-naphtyl glycidyl ether, caused sensitization in one of three workers in a chemical plant.
Chemical properties
Pale grey to brown solid with an unpleasant phenol odour. Darkens in the presence of light. Evaporates with water vapour. Soluble in ethanol, ether, benzene, chloroform and alkali solutions, insoluble in water. Precipitates violet when exposed to ferric chloride. 1-Naphthol [90-15-3] a-naphthol, 1- naphthalenol, 1-hydroxynaphthalene, C10H8O, Mr 144.16, forms colorless prisms (from toluene) which darken on exposure to air or light. The compound is steam volatile and sublimable. 1-Naphthol is reduced by sodium in liquid ammonia to give 5,6,7,8-tetrahydro-1-naphthol and is oxidized by NaOCl – FeCl3 or I2–KI to give a violet color. It is chlorinated by phosphorus pentachloride at 150℃ to give 1-chloronaphthalene, by sulfuryl chloride to give 4- chloro-1-naphthol, or by Cl2–CH3COOH to give 2,4-dichloro-1-naphthol. Similarly bromine forms 2,4-dibromo-1-naphthol, and this reaction may be used quantitatively for titration. Nitration of 1-naphthol gives complex mixtures. The crude 2,4-dinitro derivative was used in the mid-1800s as an acid yellow dye comparable to picric acid. Sulfonation at 50℃ readily yields 1-naphthol-2,4-disulfonic acid. Nitrous acid gives mainly the 2-nitroso derivative, contaminated with the 4-nitroso isomer.
The Uses of 1-Naphthol
1-Naphthol is used as a precursor in the manufacturing of various azo dyes and pharmaceuticals such as nadolol. It is used as biomarkers. It is used in analytical chemistry as Molisch's reagent (1-naphthol dissolved in ethanol) for checking the presence of carbohydrates. It plays an essential role with sodium hypobromite to detect the presence of arginine in proteins, which is called as Sakaguchi test.
Definition
ChEBI: 1-naphthol is a naphthol carrying a hydroxy group at position 1. It has a role as a genotoxin and a human xenobiotic metabolite.
What are the applications of Application
1-Naphthol is a hydroxyl-aromatic compound. It has been used as a prooxidant to analyze its ability to induce hemolysis in a zebrafish G6PD (glucose-6-phosphate dehydrogenase) deficiency model.
Preparation
2-Isopropylnaphthalene can be used to synthesize 1-naphthol by oxidation via the hydroperoxide (Hock synthesis).
Historically 1-naphthol was produced via caustic fusion of naphthalene-1-sulfonic acid, but this was superseded by the I.G. Farbenindustrie process involving hydrolysis of 1-naphthylamine with aqueous 22 % sulfuric acid at 200°C under pressure in a lead-lined autoclave. To obtain a purer product, Union Carbide developed a process based on catalytic oxidation of tetralin to 1-tetralol and 1-tetralone followed by dehydrogenation. The two-stage catalytic process is claimed to give an overall yield of 72% 1-naphthol, with an overall efficiency of 97%.
Synthesis Reference(s)
Synthetic Communications, 21, p. 379, 1991 DOI: 10.1080/00397919108016759
Journal of the American Chemical Society, 107, p. 493, 1985 DOI: 10.1021/ja00288a037
General Description
1-Naphthol, a metabolite of carbaryl and naphthanlene. It is formed by spontaneous reaction from (1R, 2S)-Naphthalene epoxide followed to form 1, 4-Dihydroxynaphthalene.
Hazard
Toxic by ingestion and skin absorption.
Flammability and Explosibility
Not classified
Contact allergens
1-Naphthol can be used in dye manufacture and is classified as a hair dye. Combined with epichlorhydrin and NaOH to form alpha-naphthyl glycidyl ether, it caused sensitization in one of three workers in a chemical plant.
Safety Profile
Poison by ingestion and intraperitoneal routes. Moderately toxic by skin contact. An experimental teratogen. Experimental reproductive effects. A severe eye and skin irritant. Mutation data reported. Ingestion of large amounts can cause nephritis, vomiting, diarrhea, circulatory collapse, anemia, convulsions, and death. Can cause kidney irritation and injury to cornea and lens of the eye. Combustible when exposed to heat or flame. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
To a 10 mL pressure vessel, aryl halide (1.00 mmol), copper (I) oxide (0.05 mol), ligand (0.05 mol), 3 M sodium hydroxide (2 mL), and organic solvent (2 mL) were added. The reaction mixture was irradiated at 160 °C for 10 min with strong stirring. The reaction mixture was allowed to cool to room temperature. The reaction mixture was filtered through a plug of celite in a fritted filter funnel and washed with ethyl acetate. The product was extracted using 30 mL of ethyl acetate for three times. The organic extract is washed three times with 10 mL water and two times with 10 mL of brine. The combined organic phases were dried over anhydrous MgSO4 and the solvent was removed under reduced pressure to afford 1-naphthol.
Preparation
1-Naphthol is prepared by two main routes.In one method, naphthalene is nitrated to give 1-nitronaphthalene, which is hydrogenated to the amine followed by hydrolysis:
C10H8 + HNO3 → C10H7NO2 + H2O
C10H7NO2 + 3H2 → C10H7NH2 + 2H2O
C10H7NH2 + H2O → C10H7OH + NH3
Toxicity
1-Naphthol has been described as "moderately toxic".
Purification Methods
Sublime 1-naphthol, then crystallise it from aqueous MeOH (charcoal), aqueous 25% or 50% EtOH, *C6H6, cyclohexane, heptane, CCl4 or H2O. Dry it over P2O5 in vacuo. The 4-nitrobenzoate has m 143o (from EtOH). [Shizuka et al. J Am Chem Soc 107 7816 1985, Beilstein 8 H 596, 6 IV 4208.]
Properties of 1-Naphthol
| Melting point: | 94-96 °C(lit.) |
| Boiling point: | 278-280 °C(lit.) |
| Density | 1.224 |
| vapor density | 4.5 (120 °C, vs air) |
| vapor pressure | 1 mm Hg ( 94 °C) |
| refractive index | 1.6224 |
| Flash point: | 125 °C |
| storage temp. | Store below +30°C. |
| solubility | Soluble in benzene, chloroform, ether and ethanol. |
| form | Crystalline Flakes |
| pka | 9.34(at 25℃) |
| color | white to off-white |
| Odor | Slight phenolic odor |
| explosive limit | 5% |
| Water Solubility | 436.7mg/L(25 ºC) |
| Sensitive | Air & Light Sensitive |
| λmax | 324nm(MeOH)(lit.) |
| Merck | 14,6383 |
| BRN | 1817321 |
| Stability: | Stable, but air and light sensitive - store under inert gas. Incompatible with strong bases, strong oxidizing agents. |
| CAS DataBase Reference | 90-15-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Naphthalenol(90-15-3) |
| EPA Substance Registry System | 1-Naphthol (90-15-3) |
Safety information for 1-Naphthol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H371:Specific target organ toxicity, single exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 1-Naphthol
| InChIKey | KJCVRFUGPWSIIH-UHFFFAOYSA-N |
1-Naphthol manufacturer
Clickchem Research LLP
Antares Chem Private Limited
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
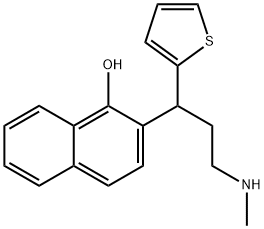
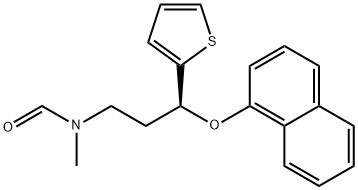
![(RS)-1-[3-(MethylaMino)-1-(2-thienyl)propyl]-2-naphthalenol](https://img.chemicalbook.in/CAS/GIF/1346599-09-4.gif)
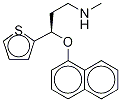
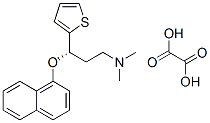
You may like
-
 1-Naphthol CAS 90-15-3View Details
1-Naphthol CAS 90-15-3View Details
90-15-3 -
 1-Naphthol (SQ) CAS 90-15-3View Details
1-Naphthol (SQ) CAS 90-15-3View Details
90-15-3 -
 Alpha Naphthol CASView Details
Alpha Naphthol CASView Details -
 Alpha Naphthol CASView Details
Alpha Naphthol CASView Details -
 1 Naphthol Chemical, 5 kg Bag, >99%View Details
1 Naphthol Chemical, 5 kg Bag, >99%View Details
90-15-3 -
 Naphthol, 25 litres Drum, Greater than 99%View Details
Naphthol, 25 litres Drum, Greater than 99%View Details
90-15-3 -
 a -NAPHTHOL 98%View Details
a -NAPHTHOL 98%View Details
90-15-3 -
 1-Naphthol AR/GRView Details
1-Naphthol AR/GRView Details
90-15-3
