1-METHYLBUTYL ACETATE
Synonym(s):2-Pentyl acetate
- CAS NO.:626-38-0
- Empirical Formula: C7H14O2
- Molecular Weight: 130.18
- MDL number: MFCD00027906
- EINECS: 210-946-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-25 18:01:13
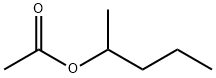
What is 1-METHYLBUTYL ACETATE?
Chemical properties
All isomers of amyl acetate are highly flammable, colorless to yellow, watery liquids.
Chemical properties
2-Pentyl acetate has an herbaceous odor.
Physical properties
Clear, colorless liquid with a fruity odor. Odor threshold concentration is 2.0 ppb (quoted, Amoore and Hautala, 1983).
Occurrence
Reportedly present in over 50 products including apple, apple juice, grape (muscat), apricot, banana, cherry, guava, melon, peach, blackberry, pear, strawberry, nectarine, potato, tomato, cocoa liquor, and vinegar.
The Uses of 1-METHYLBUTYL ACETATE
Solvent for nitrocellulose and ethyl cellulose, cements, coated paper, lacquers, leather finishes, nail enamels, plastic wood, textile sizing, and print- ing compounds.
The Uses of 1-METHYLBUTYL ACETATE
Manufacture of lacquers, artificial leather, photographic film, artificial glass, celluloid, artificial silk, and furniture polis
Preparation
Prepared from alcohols using acetic acid as an acetylating agent and clays as catalysts (patented process).
Definition
ChEBI: 2-Pentanol acetate is a carboxylic ester.
Aroma threshold values
Detection at 0.001 to 0.002 ppm (water)
General Description
Colorless to yellow watery liquid with a weak odor of bananas. Floats on water. Produces irritating vapor.
Air & Water Reactions
Highly flammable. Slightly water soluble.
Reactivity Profile
1-METHYLBUTYL ACETATE is an ester. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. 1-METHYLBUTYL ACETATE is incompatible with the following: Nitrates; strong oxidizers, alkalis & acids .
Hazard
Flammable, moderate fire risk. Toxic. Upper respiratory tract irritant.
Health Hazard
INHALATION AND INGESTION: Irritates the mucous membrane, depresses the central nervous system, and is a narcotic. Damage to kidney, liver, and lung can occur. Ingestion may irritate gastro-intestinal tract. EYES: Irritation. Skin: Irritation.
Safety Profile
Mildly toxic by inhalation. Human systemic effects by inhalation: conjunctiva irritation. Dangerous fire hazard when exposed to heat or flame; can react with oxidizing materials. Moderately explosive in the form of vapor when exposed to heat or flame. To fight fire, use alcohol foam, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
(n-isomer): Primary irritant (w/o allergic reaction), (sec-isomer) Human Data. Amyl acetates are used as industrial solvents and in the manufacturing and dry-cleaning industry; making artificial fruit-flavoring agents; cements, coated papers, lacquers; in medications as an inflammatory agent; pet repellents, insecticides and miticide. Many other uses.
Environmental Fate
Chemical/Physical. Slowly hydrolyzes in water forming acetic acid and 2-pentanol.
Shipping
UN1993 Flammable liquids, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, Technical Name Required.
Incompatibilities
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, nitrates. May soften certain plastics.
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of 1-METHYLBUTYL ACETATE
| Melting point: | -74.65°C (estimate) |
| Boiling point: | 134.85°C |
| Density | 0.8628 |
| vapor pressure | 10 at 35.2 °C (estimated, Weast, 1986) |
| refractive index | 1.3969 |
| FEMA | 4012 | 2-PENTYL ACETATE |
| solubility | Soluble in alcohol, ether (Weast, 1986), and many other organic solvents including esters and
glycols. |
| form | Liquid |
| Odor | at 100.00 %. herbal weedy musty green vegetable nut skin beany ketonic animal |
| Water Solubility | 2.2 g/L at 25 °C (Montgomery, 1989) |
| JECFA Number | 1146 |
| Henry's Law Constant | (x 10-4 atm?m3/mol):
7.7 at 25 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | NIOSH REL: TWA 125 ppm (650 mg/m3), IDLH 1,000 ppm; OSHA PEL:
TWA 125 ppm; ACGIH TLV: TWA 125 ppm with intended TWA and STEL values of 50 and
100 ppm, respectively. |
| Stability: | Volatile |
| EPA Substance Registry System | sec-Amyl acetate (626-38-0) |
Safety information for 1-METHYLBUTYL ACETATE
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H225:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P242:Use only non-sparking tools. P243:Take precautionary measures against static discharge. |
Computed Descriptors for 1-METHYLBUTYL ACETATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

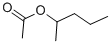
![4-(3,3-dimethylbicyclo[2.2.1]hept-2-yl)-2-methylbutyl acetate](https://img.chemicalbook.in/CAS/GIF/1146-56-1.gif)
You may like
-
 1-Methylbutyl acetate CAS 626-38-0View Details
1-Methylbutyl acetate CAS 626-38-0View Details
626-38-0 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4