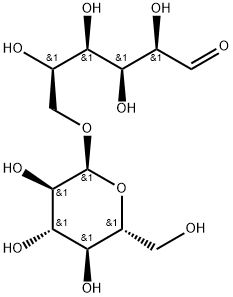
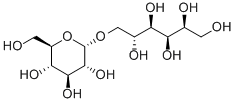
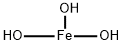(1-6)-alpha-D-Glucan reduced reaction products with iron hydroxide
- CAS NO.:1345510-43-1
- Empirical Formula: C18H34FeO16+3
- Molecular Weight: 562.29796
- Update Date: 2022-12-21 16:56:50
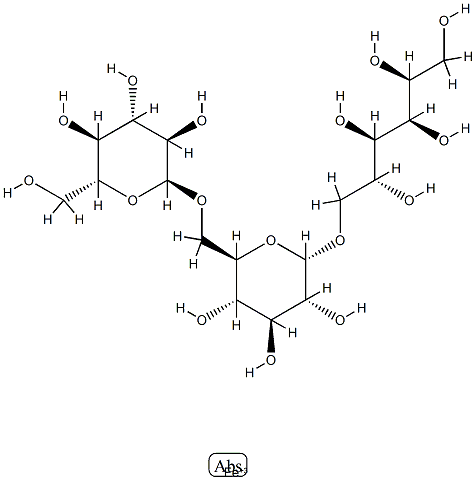
What is (1-6)-alpha-D-Glucan reduced reaction products with iron hydroxide?
Absorption
After a single 1000 mg dose, the Cmax and AUC of serum iron were 408 μg/mL and 17730 μg.h /mL, respectively. Serum ferritin concentrations reach their peak about 7 days after a single dose of intravenous ferric derisomaltose.
A note on concomitant oral iron
The absorption of oral iron is decreased when administered with intravenous iron. The administration of oral iron should be delayed until at least 5 days after the last ferric derisomaltose injection.
Toxicity
LD50 information for ferric derisomaltose is not readily available in the literature, however, the LD50 for iron sucrose (another form of intravenous iron) was 140 mg/kg in male rats. An overdose with ferric derisomaltose may lead to accumulation of stored iron, causing hemosiderosis. Symptoms may include abdominal pain, weakness, and lethargy, among others. Serum ferritin should be monitored. Employ supportive treatment including chelating agents.
Background
Iron deficiency is an extremely common condition and is the most frequent cause of anemia worldwide. Iron deficiency results when iron intake, iron stores, and loss of iron from the body do not adequately support production of erythrocytes, also known as red blood cells. Though it is generally considered non life-threatening, iron deficiency may considerably affect quality of life.
Ferric derisomaltose is a form of iron used in the treatment of iron deficiency. This drug is a complex of iron (III) hydroxide and derisomaltose. The latter is an iron carbohydrate oligosaccharide that works to release iron. Ferric derisomaltose was developed by Pharmacosmos Therapeutics ad was granted FDA approval in January 2020. Clinical trials show that it is non-inferior to iron sucrose, another form of iron that is often administered in iron deficiency, and less likely to cause serious hypersensitivity that is associated with other forms of injectable iron.
Indications
This drug is indicated for the treatment of iron deficiency anemia in adult patients who have experienced intolerance to oral iron preparations or insufficient clinical response to orally administered iron. Ferric derisomaltase is also indicated for patients with non-hemodialysis dependent chronic kidney disease. In Australia and United Kingdom, ferric derisomaltase is indicated for cases in which rapid delivery of iron is required.
Pharmacokinetics
Ferric derisomaltase increases the reticulocyte count and ultimately increases hemoglobin, treating iron deficiency anemia and its various symptoms. Parenteral iron, such as ferric derisomaltose, may cause false elevations in serum bilirubin levels and falsely reduced serum calcium.
Metabolism
Iron in the circulation is taken up by the plasma by cells of the RES. This binds proteins that form hemosiderin or ferritin, as well transferrin. Following this step, the bound iron replenishes low hemoglobin (Hb) and iron.
Safety information for (1-6)-alpha-D-Glucan reduced reaction products with iron hydroxide
Abamectin manufacturer
West Bengal Chemical Industries Ltd.
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 1345510-43-1 Ferric derisomaltose 98%View Details
1345510-43-1 Ferric derisomaltose 98%View Details
1345510-43-1 -
 1345510-43-1 99%View Details
1345510-43-1 99%View Details
1345510-43-1 -
 Ferric derisomaltose 98%View Details
Ferric derisomaltose 98%View Details
1345510-43-1 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4