(+)-Ledene
Synonym(s):(1S,2R,4R,11R)-3,3,7,11-Tetramethyltricyclo[6.3.0.02.4]undec-7-ene
- CAS NO.:21747-46-6
- Empirical Formula: C15H24
- Molecular Weight: 204.35
- MDL number: MFCD00042613
- EINECS: 244-565-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-27 16:58:12
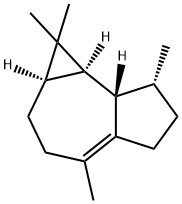
What is (+)-Ledene?
Description
(+)-Ledene, also called leden or viridiflorene, is an organic compound that belongs to the group of 5, 10-cycloaromadendrane sesquiterpenoids. These compounds are derived from the aromadendrane skeleton through a cyclization reaction between carbon atoms 5 and 10. (+)-Ledene is mainly found in saliva and is considered to have low solubility in water and a relatively neutral nature. In cellular environments, (+)-ledene is predominantly present in the cell membrane (predicted based on logP) and cytoplasm.
The Uses of (+)-Ledene
(+)-Ledene is an essential oil that is synthesized by viridiflorene synthase. It has been studied as an antioxidant, antimicrobial, antibiofilm and to treat various cancers.
Definition
ChEBI: Viridiflorene is a carbotricyclic sesquiterpene obtained from several natural sources, including Australian Tea Tree oil (Melaleuca alternifolia.) It is a sesquiterpene and a carbotricyclic compound.
General Description
(+)-Ledene is a naturally occurring sesquiterpene that can be prepared from (+)-aromadendrene via isomerization.
References
[1] DUC N. TRAN Prof.??Dr. N C. Biomimetic Synthesis of (+)-Ledene, (+)-Viridiflorol, (?)-Palustrol, (+)-Spathulenol, and Psiguadial A, C, and D via the Platform Terpene (+)-Bicyclogermacrene[J]. Chemistry - A European Journal, 2014. DOI:10.1002/chem.201403082.
[2] S. L. GWALTNEY K. J S S T Sakata. Bridged to Fused Ring Interchange. Methodology for the Construction of Fused Cycloheptanes and Cyclooctanes. Total Syntheses of Ledol, Ledene, and Compressanolide[J]. The Journal of Organic Chemistry, 1996. DOI:10.1021/jo961005a.
Properties of (+)-Ledene
| Melting point: | 269°C |
| Boiling point: | 268-270 °C (lit.) |
| Density | 0.927 g/mL at 20 °C (lit.) |
| refractive index | n |
| storage temp. | Refrigerator |
| solubility | Chloroform (Sparingly), Ethanol (Slightly) |
| form | Oil |
| color | Colourless |
| optical activity | [α]20/D +68±2°, c = 10% in ethanol |
| BRN | 2554786 |
Safety information for (+)-Ledene
Computed Descriptors for (+)-Ledene
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 6-Aminouracil (or) 4-Amino-2,6- dihydroxypyrimidine, (or) 6-Amino2,4-pyrimidinediol 99%View Details
873-83-6 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 207557-35-5 99%View Details
207557-35-5 99%View Details
207557-35-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1