(+)-BORNEOL
Synonym(s):endo-(1R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
- CAS NO.:464-43-7
- Empirical Formula: C10H18O
- Molecular Weight: 154.25
- MDL number: MFCD00066427
- EINECS: 207-352-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
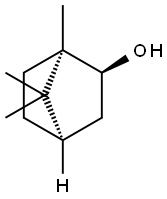
What is (+)-BORNEOL?
Chemical properties
Almost insoluble in water, poorly soluble in Propylene glycol, very soluble in alcohol, miscible with most perfume oils. Dry-camphoraceous, woody-peppery odor. The dryness is characteristic and constitutes the main difference from the odor of isoBorneol. The odor of Borneol is often compared to that of a good grade Rosemary oil (in which Borneol is present).
The Uses of (+)-BORNEOL
(+)-Borneol has been used to study its antiapoptotic, antioxidative and neuroprotective effect in human neuroblastoma cells (SH-SY5Y).
What are the applications of Application
(+)-Borneol is originally isolated from various aromatic plants
Definition
ChEBI: (+)-borneol is a borneol. It is an enantiomer of a (-)-borneol.
What are the applications of Application
(+)-Borneol has a woody, somewhat minty and slightly burning taste. This alcohol blends excellently with Olibanum products for “incense” type fragrances, and it forms a good background in many herbaceous-camphoraceous fragrances, Lavender- and Citrus cologne types, many tyDes of Room-freshener fragrance, Pine odors, etc.
General Description
(+)-Borneol, a bicyclic monoterpene, is found in the essential oils of medicinal plants and is commonly used in traditional Chinese medicine for analgesia and anaesthesia.
Anticancer Research
Wild asparagus root is known as “tian men dong” in traditional Chinese medicine. Itis used to relieve asthma, suppress coughing, and promote expectoration (Huang1998). It is held to be sweet and bitter in flavour and cold in nature, nourishing thelungs and moistening dryness (McNamara and Song 1995). Though studies conductedon asparagus root to examine its biological effects have only been conductedon animals, the evidence so far shows anticancer activity against leukaemia and lungcancer by means of the inhibition of tumour necrosis factor alpha (Huang et al. 2008).
Purification Methods
It can be steam distilled, the distillate is extracted into Et2O, the extract dried with Drierite and evaporated. The residue is then recrystallised from boiling EtOH (charcoal) or pet ether. [Clark & Read J Chem Soc 1773 1934, Beilstein 6 III 295, 6 IV 281.]
Properties of (+)-BORNEOL
| Melting point: | 206-209 °C (lit.) |
| alpha | 36 º (C=5 IN ETOH) |
| Boiling point: | 237.64°C (rough estimate) |
| Density | 0.8704 (rough estimate) |
| refractive index | 1.4723 (estimate) |
| FEMA | 2157 | BORNEOL |
| Flash point: | 150 °F |
| storage temp. | 2-8°C |
| solubility | DMF: 25 mg/ml; DMF:PBS(pH7.2) (1:2): 0.33 mg/ml; DMSO: 16 mg/ml; Ethanol: 16 mg/ml |
| pka | 15.36±0.60(Predicted) |
| form | neat |
| Odor | at 10.00 % in dipropylene glycol. pine camphor earthy |
| optical activity | [α]20/D 37.0±2.0°, c = 5 in ethanol |
| Water Solubility | 0.74g/L(25 ºC) |
| BRN | 2038056 |
| CAS DataBase Reference | 464-43-7(CAS DataBase Reference) |
Safety information for (+)-BORNEOL
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H228:Flammable solids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for (+)-BORNEOL
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 (+)-Borneol CASView Details
(+)-Borneol CASView Details -
 (+)-Borneol CAS 464-43-7View Details
(+)-Borneol CAS 464-43-7View Details
464-43-7 -
 (+)-Borneol CAS 464-43-7View Details
(+)-Borneol CAS 464-43-7View Details
464-43-7 -
 (+)-Borneol solution CAS 464-43-7View Details
(+)-Borneol solution CAS 464-43-7View Details
464-43-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1