2-(2-Bromophenyl)pyrrolidine
- CAS NO.:129540-24-5
- Empirical Formula: C10H12BrN
- Molecular Weight: 226.11
- MDL number: MFCD02663433
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-18 09:20:08
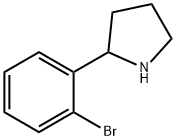
What is 2-(2-Bromophenyl)pyrrolidine?
Description
2-(2-Bromophenyl)pyrrolidine is a drug that has been studied for its effects on cyclophilins, which are involved in the regulation of cell division. The crystal structure of 2-bromopyrrolidine has been determined by x-ray crystallography and computational simulations. The distal phenyl group of the molecule binds to the active site of cyclophilin A, and this binding prevents ATP from binding, leading to inhibition of cell division. This compound also inhibits cyclophilin B and D. 2-(2-Bromophenyl)pyrrolidine is a member of the family of drugs called pyrazolopyrimidines that target cyclophilins for treatment of cancer and inflammatory diseases.
The Uses of 2-(2-Bromophenyl)pyrrolidine
2-(2-Bromophenyl)pyrrolidine acts as a reagent in the preparation of pyrroloisoquinolinone derivatives which are potential antidepressants.
Reactions
2-(2-Bromophenyl)pyrrolidine is an important building block for the synthesis of various organic compounds. It could be employed to couple with ethyl 2-{[(cyclopropylmethyl)[(4-nitrophenyl)methyl]carbamoyl]amino}acetate to synthesize 3-{2-[(2S)-2-(2-bromophenyl)pyrrolidin-1-yl]-2-oxoethyl}-1-[(1-methyl-1H-1,2,3-triazol-4-yl)methyl]-1-[(4-nitrophenyl)methyl]urea (1), which is an important intermediate for the synthesis of tri-vectors Cyps ligands. Through the coupling of the amine, it can also synthesize tert-butyl 2-(2-bromophenyl)pyrrolidine-1-carboxylate (2) with Boc2O.
Synthesis
5-(2-Bromophenyl)-3,4-dihydro-2H-pyrrole (393 mmol, 88 g) was dissolved in methanol (1300 mL), then the acetic acid (330 mL) was added and the solution cooled to -65° C. under a nitrogen atmosphere. Sodium borohydride (589 mmol, 22.28 g) was added portionwise over 1 hour. The reaction was stirred at -65° C. for 30 minutes, then the cooling bath was removed and the reaction mixture temperature was allowed to rise to room temperature. The bulk of the methanol was removed under vacuum then 5N HCl (950 mL) was added and the solution extracted with ether (2×500 mL). The aqueous solution was then basified with sodium hydroxide pellets (310 g) with ice-bath cooling, maintaining the reaction temperature less than 30° C. The basified aqueous was then extracted with ethyl acetate (3×800 mL), the combined organics washed with brine (800 mL), dried with sodium sulfate, evaporated and chromatographed on 1 Kg of silica gel eluting with 19:1 to 9:1 methylene chloride-ethanol to give 2-(2-bromophenyl)pyrrolidine (68.5 g).
Properties of 2-(2-Bromophenyl)pyrrolidine
| Boiling point: | 274℃ |
| Density | 1.369 |
| Flash point: | 120℃ |
| storage temp. | 2-8°C(protect from light) |
| pka | 9.45±0.10(Predicted) |
Safety information for 2-(2-Bromophenyl)pyrrolidine
Computed Descriptors for 2-(2-Bromophenyl)pyrrolidine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran








You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4