- Price
Supplier Type
- Manufacturer
Supplier Region
- Gujarat(1)
Purity
- United States Pharmacopeia (USP) Reference Standard
- European Pharmacopoeia (EP) Reference Standard
- certified reference material, pharmaceutical secondary standard
- 90 % Above
Package
- 25mg
- 100mg
- 250mg
- 50010500 mg and 1.0 gm
- 5mg
- 10mg
- 50mg
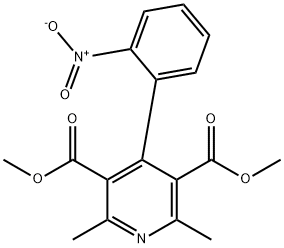
OXIDIZED NIFEDIPINE
- CAS:67035-22-7
- MF:C17H16N2O6
- MW:344.32
- Melting point::
- 100-105 °C
- Boiling point::
- 443.7±45.0 °C(Predicted)
- Density :
- 1.284±0.06 g/cm3(Predicted)
- storage temp. :
- 2-8°C
- solubility :
- Chloroform (Slightly), Methanol (Slightly)
- pka:
- 1.55±0.29(Predicted)
- form :
- powder
- color :
- yellow
- Symbol(GHS)

GHS05
- Signal word
- Danger
- Hazard statements
- H318
- Precautionary statements
- P280-P305+P351+P338+P310
NIFEDIPINE EP IMP A 67035-22-7
Manufacturer
Note:Purity: 90 % Above | Package: 25 mg, 100 mg, 250 mg, 500 mg and 1.0 gm
1Y
Gujarat
Phone: +91-8140031133
Tel: +91-8140031133
Whatsapp: +91- 8140031133
Email: mayur@clickchemresearch.com
Product catalog:
434
Nifedipine Nitrophenylpyridine Analog 67035-22-7
Pack Size
Purity
Availability
Price
25mg
United States Pharmacopeia (USP) Reference Standard
 0
0
₹340393
Update time:2024-08-21