- Price
Supplier Type
- Reagent
- Trader
- contract manufacturer
- custom synthesis
Supplier Region
- Gujarat(3)
- New Delhi(1)
- Maharashtra(3)
- Mumbai(1)
- Tamil Nadu(1)
Purity
- United States Pharmacopeia (USP) Reference Standard
- Pharmaceutical Secondary Standard
- Greater than 99%
- Certified Reference Material
- >99%
- 99%
- >98.0%(T)(HPLC)
- 95%
Package
- 10mg
- 20mg
- 50mg
- 200MG
- 1g
- 18%
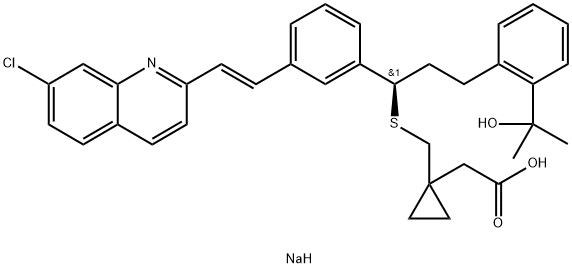
Montelukast sodium
- CAS:151767-02-1
- MF:C35H37ClNNaO3S
- MW:610.18
- EINECS:604-813-7
- Melting point::
- 115 °C(dec.)
- storage temp. :
- 2-8°C
- solubility :
- DMSO: ≥8mg/mL at 60°C
- form :
- powder
- color :
- white to tan
- optical activity:
- [α]/D +90 to +106° in methanol (c=1)
- Merck :
- 14,6258
- Stability::
- Stable for 1 year from date of purchase as supplied. Solutions in DMSO, distilled water, or ethanol may be stored at -20°C for up to 1 month.
- InChIKey:
- LBFBRXGCXUHRJY-HKHDRNBDSA-M
- Symbol(GHS)


GHS05,GHS08
- Signal word
- Danger
- Hazard statements
- H318-H361fd
- Precautionary statements
- P280-P305+P351+P338+P310

Montelukast sodium 151767-02-1
Trader
Gujarat
Phone: 91-9033667967
Whatsapp: 91-9033667967
Web: festivapharma.com
Email: sales@festivapharma.com
Product catalog:
301

Montelukast sodium 151767-02-1
Trader
contract manufacturer
Maharashtra
Phone: 91-9833030098
Tel: 91-22-28607070
Whatsapp: 91-9833030098
Web: www.bazayan.ch
Email: info@bazayan.ch
Product catalog:
366

Montelukast sodium 151767-02-1
Trader
Mumbai
Phone: 91-9820001553
Tel: 91-22-25004510
Whatsapp: 91-9820001553
Web: www.umabrothers.com
Email: umabros@hotmail.com
Product catalog:
55

Montelukast sodium 151767-02-1
Trader
Maharashtra
Phone: 91-9321294806
Tel: 91-49798100
Whatsapp: 91-9321294806
Web: www.geminiexports.in
Email: samit1@geminiexports.in
Product catalog:
53

Montelukast sodium 151767-02-1
Trader

Montelukast sodium 151767-02-1
contract manufacturer
custom synthesis
Maharashtra
Tel: 91-22-66707500
Web: www.ionspharma.com
Email: ions@ionspharma.com
Product catalog:
79