Supplier Type
- contract manufacturer
- Manufacturer
- custom synthesis
- Trader
- Reagent
Supplier Region
- Hyderabad(7)
- Ahmedabad(1)
- Maharashtra(38)
- Telangana(10)
- Gujarat(23)
- Karnataka(6)
- Madhya Pradesh(1)
- Mumbai(9)
- West Bengal(1)
- Kerela(1)
- Tamil Nadu(1)
Purity
- IP/USP/BP/EP
- IP/BP-EP/USP
- IP/BP/EP/USP
- IP/BP/EP
- Complies as per Monograph
- BP/EP/USP
- 99%
- 98%
- >98%
- 96%
- 95%
- 90 % Above
- 80%
- 0.98
Package
- 0As per requirement
- 25mg
- 50mg
- 100mg
- 250mg
- 500mg
- 1g
- 5g
- 10g
- 25g
- 50g
- 100g
- 250g
- 500g
- 1kg
- 2Kg
- 5kg
- 10kg
- 25125kg and 1 MT
- 50010500 mg and 1.0 gm
- 100500100mg or 500 mg
- 50kg
- 100kg
- 1000kg
- 1 kg
- 5 kg
- 10mt
- 10 kg
- 1001100mg and 1 g
- 25kg
- 1MT
- 200KGS HDPE DRUM
- 2.55022e+01025/50/220 KGS HDPE DRUM / 1060 KGS IBC TANKS
- 20KG
-
3,5,3',5'-Tetraiodo Thyroaldehyde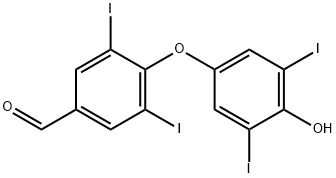
2016-06-0
-
3,5-DIIODO-4'-(4-HYDROXYPHENOXY)BENZOIC ACID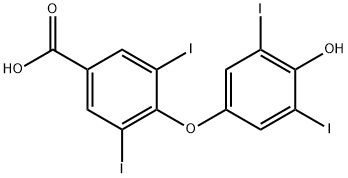
2055-97-2
-
3,5-Diiodo-L-thyronine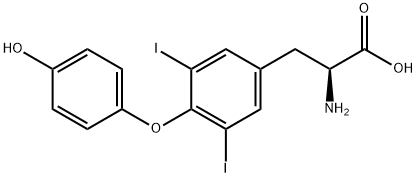
1041-01-6
-
Acyclovir Impurity I: 7,9'-[Ethylene-bis(oxymethylene)] Bis (2-amino-1-9-dihydro-6H-purin-6-one)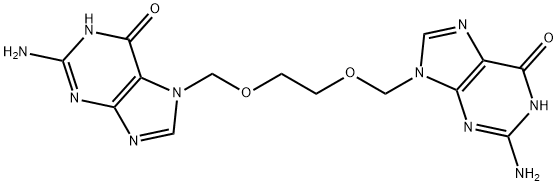
1797832-75-7
-
REVERSE T3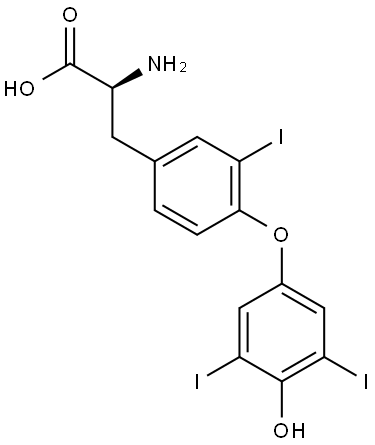
5817-39-0
-
Ivermectin EP Impurity I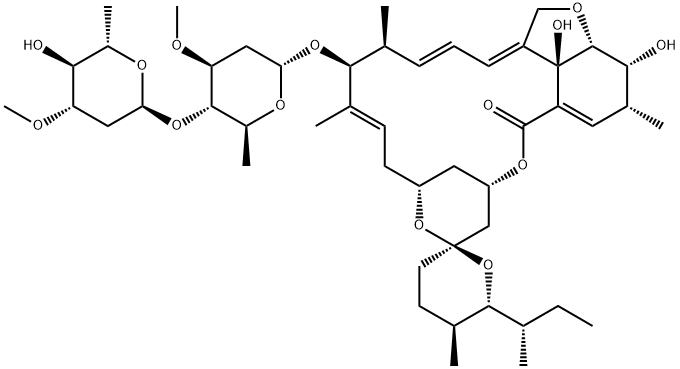
1135339-49-9
-
Omeprazole Sulfone N-Oxide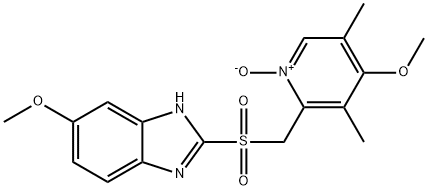
158812-85-2
-
Betamethasone EP Impurity I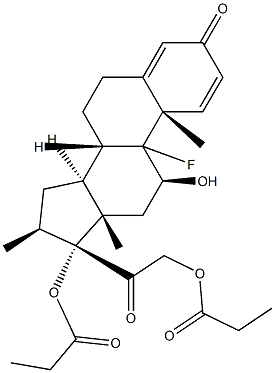
185613-69-8
-
16α-Butyloxyprednisolone (Budenoside IMpurity)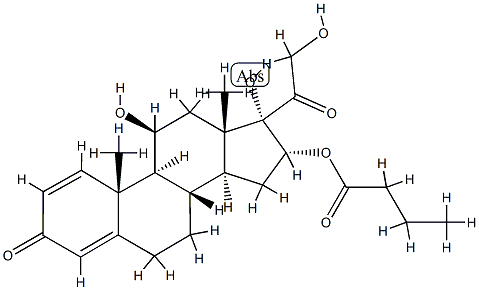
113930-13-5
-
2,3,5,6-Tetrafluorophenol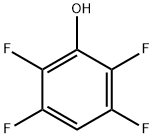
769-39-1
Levothyroxine EP Impurity I 2016-06-0
Levothyroxine EP Impurity -E 1041-01-6
100mg and 1 g 50mg 25mg
Omeprazole EP Impurity I Omeprazole USP RC I 158812-85-2
Levothyroxine sodium 55-03-8 / 50809-32-0
MICONAZOLE EP IMP I 24169-00-4
Levothyroxine sodium 55-03-8 / 50809-32-0
Levothyroxine sodium 55-03-8 / 50809-32-0
Telmisartan EP impurity B 1026353-20-7


















