CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Light yellow powder; [MSDSonline] |
|---|---|
| Ionization Efficiency | Positive |
| Kovats Retention Index | 1304.2 1314.5 |
| Chemical Classes | Nitrogen Compounds -> Other Aromatics (Nitrogen) |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
COMPUTED DESCRIPTORS
| Molecular Weight | 151.12 g/mol |
|---|---|
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 151.026943022 g/mol |
| Monoisotopic Mass | 151.026943022 g/mol |
| Topological Polar Surface Area | 62.9 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 164 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
Description
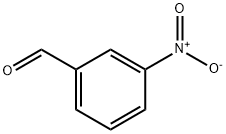
3-Nitrobenzaldehyde, also known as meta-nitrobenzaldehyde or m-nitrobenzaldehyde, is an organic aromatic compound distinguished by the presence of a nitro group that is meta-substituted to an aldehyde functional group. This compound is primarily synthesized through a process known as mono-nitration, where benzaldehyde reacts with nitric acid to yield 3-nitrobenzaldehyde as the main product. This chemical synthesis is significant in organic chemistry, as it allows for the creation of a compound with distinct properties due to the meta positioning of the nitro group relative to the aldehyde.
RELATED SUPPLIERS
All suppliers(6)