9011-14-7
Product Name:
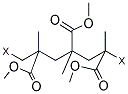
Poly(methyl methacrylate)
Formula:
(C5H8O2)x
Synonyms:
Isotactic methyl methacrylate polymer;Isotactic PMMA;Isotactic poly(methyl methacrylate);PMMA;Poly(methacrylic acid methyl ester)
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Methyl methacrylate monomer appears as a clear colorless liquid. Slightly soluble in water and floats on water. Vapors heavier than air. Vapors irritate the eyes and respiratory system. Containers must be heavily insulated or shipped under refrigeration. An inhibitor such as hydroquinone, hydroquinone methyl ester and dimethyl t-butylphenol is added to keep the chemical from initiating polymerization. The chemical may polymerize exothermically if heated or contaminated with strong acid or base. If the polymerization takes place inside a container, the container may rupture violently. Used to make plastics. |
|---|---|
| Color/Form | Colorless volatile liquid |
| Odor | Characteristic quality: sulfur-like, sweet, sharp; hedonic tone: unpleasant |
| Boiling Point | 213.8 °F at 760 mmHg (NTP, 1992) |
| Melting Point | -54 °F (NTP, 1992) |
| Flash Point | 50 °F (NTP, 1992) |
| Solubility | 1 to 10 mg/mL at 63.5 °F (NTP, 1992) |
| Density | 0.945 at 68 °F (USCG, 1999) - Less dense than water; will float |
| Vapor Density | 3.45 (NTP, 1992) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 40 mmHg at 77.9 °F (NTP, 1992) |
| LogP | log Kow = 1.38 |
| Autoignition Temperature | 790 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits acrid smoke and irritating fumes. |
| Heat of Combustion | -11,400 BTU/lb = 6,310 cal/g = -264X10+5 J/kg (estimated) |
| Heat of Vaporization | 36.0 kJ/mol at 100.5 °C |
| Surface Tension | 0.028 N/m at 20 °C. |
| Ionization Potential | 9.70 eV |
| Polymerization | May polymerize if subjected to heat, polymerization catalysts (eg, azoisobutyronitrile, dibenzoyl peroxide, di-tert-butyl peroxide, propionaldehyde), strong oxidizers, or ultraviolet light. |
| Odor Threshold | Odor Threshold Low: 0.01 [mmHg] Odor Threshold High: 0.46 [mmHg] Detection odor threshold from AIHA (mean = 0.049 ppm) |
| Refractive Index | Index of refraction: 1.4142 at 20 °C |
| Relative Evaporation Rate | 3.1 (Butyl acetate = 1) |
| Kovats Retention Index | 723 672 696 700 694 677 699 677 677 677 710 699 696 694.1 696.1 696 696 699 692.9 677 699 |
| Other Experimental Properties | Conversion factor: 1 ppm in air = 4.1 mg/cu m |
| Chemical Classes | Plastics & Rubber -> (Meth)acrylates |
COMPUTED DESCRIPTORS
| Molecular Weight | 100.12 g/mol |
|---|---|
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 100.052429494 g/mol |
| Monoisotopic Mass | 100.052429494 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 94.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Methyl methacrylate monomer appears as a clear colorless liquid. Slightly soluble in water and floats on water. Vapors heavier than air. Vapors irritate the eyes and respiratory system. Containers must be heavily insulated or shipped under refrigeration. An inhibitor such as hydroquinone, hydroquinone methyl ester and dimethyl t-butylphenol is added to keep the chemical from initiating polymerization. The chemical may polymerize exothermically if heated or contaminated with strong acid or base. If the polymerization takes place inside a container, the container may rupture violently. Used to make plastics.
