87-89-8
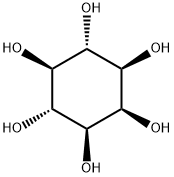
Product Name:
Inositol
Formula:
C6H12O6
Synonyms:
myo-Inositol;ELISA;Hexahydroxycyclohexane, Cyclohexanehexol, meso-Inositol, Myo-Inosit;i-Inositol;meso-Inositol
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dry Powder |
|---|---|
| Boiling Point | 291.33 ºC |
| Melting Point | 224.5 ºC |
| Solubility | 0.17 mg/ml |
| LogP | -2.08 |
| Collision Cross Section | 148.2 Ų [M+Na]+ [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)] |
| Kovats Retention Index | 1974 1981 |
| Chemical Classes | Biological Agents -> Monosaccharides and Derivatives |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
COMPUTED DESCRIPTORS
| Molecular Weight | 180.16 g/mol |
|---|---|
| XLogP3 | -3.7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 180.06338810 g/mol |
| Monoisotopic Mass | 180.06338810 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 104 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Myo-inositol is an inositol having myo- configuration. It has a role as a member of compatible osmolytes, a nutrient, an EC 3.1.4.11 (phosphoinositide phospholipase C) inhibitor, a human metabolite, a Daphnia magna metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite.
