83729-01-5
Product Name:
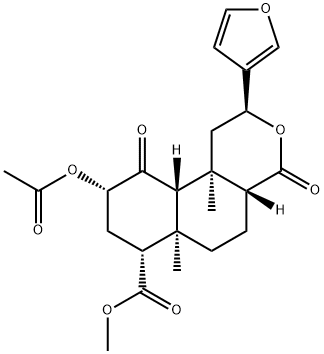
SALVINORIN A
Formula:
C23H28O8
Synonyms:
(2S,4aR,6aR,7R,9S,10aS,10bR)-9-(acetyloxy)-2-(3-furanyl)dodechydro-6a,10b-dimethyl-4,10-dioxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Colorless solid; [Merck Index] White film; [Sigma-Aldrich MSDS] |
|---|---|
| Color/Form | Colorless crystals from methanol |
| Melting Point | 238-240 °C; also reported as 242-244 °C |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Optical Rotation | Specific optical rotation: -45.3 deg at 22 °C/D ( c = 8.530 CHCl3); -41 °C at 25 °C/D ( c = 1 in CHCl3) |
| Decomposition | Hazardous decomposition products formed under fire conditions - Carbon oxides. |
| Chemical Classes | Biological Agents -> Plant Toxins |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P337+P313:IF eye irritation persists: Get medical advice/attention. P403+P235:Store in a well-ventilated place. Keep cool. |
COMPUTED DESCRIPTORS
| Molecular Weight | 432.5 g/mol |
|---|---|
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 432.17841785 g/mol |
| Monoisotopic Mass | 432.17841785 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 792 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Salvinorin A is an organic heterotricyclic compound and an organooxygen compound. It has a role as a metabolite and a oneirogen.
