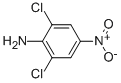
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Dichloran appears as yellow crystals or solid. Slight aniline odor. Used as a fungicide. Insoluble in water, but often formulated as a wettable powder (easily dispersed in water). |
|---|---|
| Color/Form | Yellow needles from alcohol and acetic acid |
| Odor | Odorless |
| Boiling Point | at 0.27kPa: 130 °C |
| Melting Point | 374 to 378 °F (NTP, 1992) |
| Solubility | less than 0.1 mg/mL at 77 °F (NTP, 1992) |
| Density | 0.28 (bulk) |
| Vapor Pressure | 1.2e-06 mmHg at 68 °F (NTP, 1992) |
| LogP | log Kow = 2.80 at 25 °C |
| Stability/Shelf Life | STABLE TO HYDROLYSIS & OXIDATION |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen chloride and nitrogen oxides/. |
| Corrosivity | NON-CORROSIVE |
| Dissociation Constants | pKa = 3.31 |
| Collision Cross Section | 132.54 Ų [M+H]+ 129.45 Ų [M-H]- |
| Kovats Retention Index | 1716 1699.4 1696.7 1709.7 1680 1682.1 1693.5 1691.3 |
| Other Experimental Properties | BROWNISH-YELLOW /TECHNICAL PRODUCT, PURITY MORE THAN 90%/ |
| Chemical Classes | Pesticides -> Fungicides |
COMPUTED DESCRIPTORS
| Molecular Weight | 207.01 g/mol |
|---|---|
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 205.9649828 g/mol |
| Monoisotopic Mass | 205.9649828 g/mol |
| Topological Polar Surface Area | 71.8 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 173 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Dichloran appears as yellow crystals or solid. Slight aniline odor. Used as a fungicide. Insoluble in water, but often formulated as a wettable powder (easily dispersed in water).