80418-24-2
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | White to off-white powder |
|---|---|
| Solubility | In water, 1 mg/mL, clear colorless solution |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Hazardous decomposition products formed under fire conditions. - Carbon oxides Other decomposition products - no data available |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 933.1 g/mol |
|---|---|
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 12 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 12 |
| Exact Mass | 932.53446570 g/mol |
| Monoisotopic Mass | 932.53446570 g/mol |
| Topological Polar Surface Area | 298 Ų |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1670 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 25 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
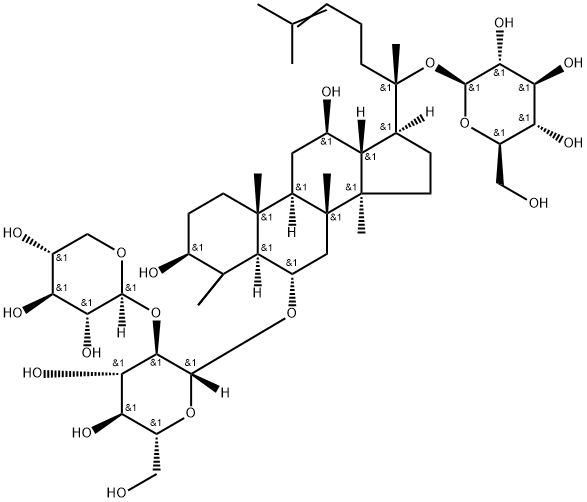
Notoginsenoside R1 is a ginsenoside found in Panax notoginseng that is dammarane which is substituted by hydroxy groups at the 3beta, 6alpha, 12beta and 20 pro-S positions, in which the hydroxy groups at positions 6 and 20 have been converted to the corresponding beta-D-xylopyranosyl-(1->2)-beta-D-glucopyranoside and beta-D-glucopyranoside respectively, and in which a double bond has been introduced at the 24-25 position. It has a role as a plant metabolite, an antioxidant, a neuroprotective agent, an apoptosis inducer and a phytoestrogen. It is a beta-D-glucoside, a 12beta-hydroxy steroid, a 3beta-hydroxy steroid, a disaccharide derivative, a ginsenoside, a tetracyclic triterpenoid and a 3beta-hydroxy-4,4-dimethylsteroid. It derives from a hydride of a dammarane.