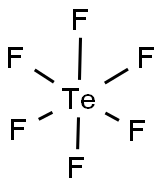
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Tellurium hexafluoride is a colorless gas with a repulsive odor. It is very toxic by inhalation and skin absorption. When heated to high temperatures, it may emit toxic fluoride and tellurium fumes. It is heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket. It reacts with water to yield toxic vapors. |
|---|---|
| Color/Form | Colorless gas |
| Odor | Repulsive odor |
| Boiling Point | 95.9 °F at 760 mmHg (EPA, 1998) |
| Melting Point | -35.7 °F (EPA, 1998) |
| Solubility | Decomposes (NIOSH, 2023) |
| Density | 2.499 @ -10 °C /Liquid/ |
| Vapor Density | 2.499 at 14 °F (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | Greater than 760 mmHg (EPA, 1998) |
| Stability/Shelf Life | DECOMPOSES IN HOT & COLD WATER, ACID & ALKALI |
| Decomposition | TOXIC GASES AND VAPORS (SUCH AS HYDROGEN FLUORIDE) MAY BE RELEASED WHEN TELLURIUM HEXAFLUORIDE DECOMPOSES. |
| Corrosivity | Does not attack glass when pure; corrodes mercury |
| Other Experimental Properties | Reacts with H2O |
| Chemical Classes | Toxic Gases & Vapors -> Corrosive Gases |
COMPUTED DESCRIPTORS
| Molecular Weight | 241.6 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 0 |
| Exact Mass | 243.8966417 g/mol |
| Monoisotopic Mass | 243.8966417 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 7 |
| Formal Charge | 0 |
| Complexity | 62.7 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Tellurium hexafluoride is a colorless gas with a repulsive odor. It is very toxic by inhalation and skin absorption. When heated to high temperatures, it may emit toxic fluoride and tellurium fumes. It is heavier than air. Under prolonged exposure to fire or intense heat the containers may violently rupture and rocket. It reacts with water to yield toxic vapors.