CHEMICAL AND PHYSICAL PROPERTIES
| Decomposition | When heated to decompostion it emits toxic vapors of /oxides of sulfur, oxides of nitrogen, and fluorine/. |
|---|---|
| Collision Cross Section | 170.13 Ų [M-H]- |
| Other Experimental Properties | When heated to decomposition it emits toxic vapors of SOx, Nox, and F(-) |
COMPUTED DESCRIPTORS
| Molecular Weight | 499.15 g/mol |
|---|---|
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 20 |
| Rotatable Bond Count | 7 |
| Exact Mass | 498.9534782 g/mol |
| Monoisotopic Mass | 498.9534782 g/mol |
| Topological Polar Surface Area | 68.5 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 730 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
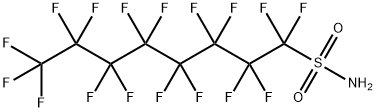
Perfluorooctanesulfonamide is a perfluorinated compound that is perfluorooctane in which one of the terminal fluorines has been replace by a sulfamoyl group. It has a role as a persistent organic pollutant. It is a sulfonamide and a perfluorinated compound.