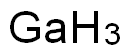CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Gallium is a silvery-white liquid at room temperature. Ingestion of this material may be toxic. It is corrosive to aluminum. If exposed to high temperatures, gallium may emit toxic fumes which may form a corrosive alkaline solution with water. It is soluble in most acids and alkalis. It is used as a semiconductor material. |
|---|---|
| Color/Form | Grayish metal, possesses a greenish-blue reflection or silver-like when molten; has a crystalline ortho-rhombic texture |
| Boiling Point | Approx 2400 °C |
| Melting Point | 29.78 °C |
| Solubility | Sol in acid, alkali; slightly sol in mercury |
| Density | 6.0947 @ 29.8 °C (liquid); 5.9037 @ 29.65 °C (solid) |
| Vapor Pressure | 1 Pa @ 1037 °C; 10 Pa @ 1175 °C; 100 Pa @ 1347 °C... |
| Viscosity | 1.819 cP @ 32 °C (dynamic) |
| Corrosivity | At a temperature of 500-100 °C or more, gallium is very corrosive to most metals. |
| Heat of Vaporization | 254 kJ/mol @ approx 2400 °C |
| Surface Tension | 709 dyn/cm @ 30 °C |
| Relative Evaporation Rate | Zero |
| Other Experimental Properties | Reacts with most metals at high temperatures |
| Chemical Classes | Metals -> Elements, Metallic |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H290:Corrosive to Metals H302:Acute toxicity,oral H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P234:Keep only in original container. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P390:Absorb spillage to prevent material damage. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
COMPUTED DESCRIPTORS
| Molecular Weight | 69.723 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 68.92557 g/mol |
| Monoisotopic Mass | 68.92557 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Gallium is a silvery-white liquid at room temperature. Ingestion of this material may be toxic. It is corrosive to aluminum. If exposed to high temperatures, gallium may emit toxic fumes which may form a corrosive alkaline solution with water. It is soluble in most acids and alkalis. It is used as a semiconductor material.