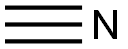| Physical Description |
Colorless or pale-blue liquid or gas (above 78 degrees F) with a bitter, almond-like odor. [Note: Often used as a 96% solution in water.] [NIOSH] |
| Color/Form |
Colorless gas or liquid |
| Odor |
Bitter almond odor |
| Taste |
Bitter, burning taste |
| Boiling Point |
25.63 °C |
| Melting Point |
-13.28 °C |
| Flash Point |
0 °F (-18 °C) (Closed cup) |
| Solubility |
Miscible with water |
| Density |
0.6875 g/cu cm at 20 °C |
| Vapor Density |
0.932 (Air = 1) |
| Vapor Pressure |
742.0 [mmHg] |
| LogP |
log Kow = -0.25 |
| Henry's Law Constant |
Henry's Law constant = 1.33X10-4 atm-cu m/mol at 25 °C |
| Stability/Shelf Life |
Soln sensitive to light. |
| Autoignition Temperature |
1000 °F (538 °C) |
| Corrosivity |
Although HCN is a weak acid and normally not considered corrosive, it has a corrosive effect under two special conditions: (1) water solutions of HCN cause transcrystalline stress-cracking of carbon steels under stress even at room temperature and in dilute solution; (2) water solutions of HCN containing sulfuric acid as a stabilizer severely corrode steel above 40 °C and stainless steels above 80 °C. |
| Heat of Combustion |
642 kJ/mol |
| Heat of Vaporization |
25.2 kJ/mol |
| pH |
Very weak acid (does not redden litmus) |
| Ionization Potential |
13.60 eV |
| Polymerization |
When not absolutely pure or stabilized, hydrogen cyanide polymerizes spontaneously with explosive violence. |
| Odor Threshold |
Odor Threshold Low: 2.0 [mmHg] Odor Threshold High: 10.0 [mmHg] |
| Refractive Index |
Index of refraction: 1.2614 at 20 °C/D |
| Dissociation Constants |
pKa = 9.2 |
| Kovats Retention Index |
319.9 320 |
| Other Experimental Properties |
Burns in air with a blue flame (acid) |
| Chemical Classes |
Toxic Gases & Vapors -> Chemical Asphyxiants |