7240-90-6
Product Name:
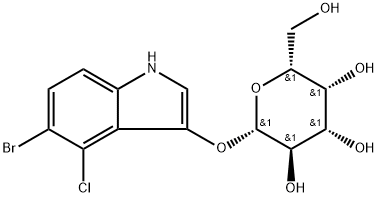
5-Bromo-4-chloro-3-indolyl-beta-D-galactoside
Formula:
C14H15BrClNO6
Synonyms:
X-Gal;BCIG;bromo-4-chloro-3-indolyl-β-d-galactopyranoside, 5-;5-Bromo-4-chloro-3-indolyl β-D -galactopyranoside;5-Bromo-4-chloro-3-indolyl β-D -galactoside
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid; [Merck Index] White powder; [Sigma-Aldrich MSDS] |
|---|---|
| Chemical Classes | Other Uses -> Biochemical Research |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P330:Rinse mouth. P363:Wash contaminated clothing before reuse. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P403:Store in a well-ventilated place. |
COMPUTED DESCRIPTORS
| Molecular Weight | 408.63 g/mol |
|---|---|
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 406.97713 g/mol |
| Monoisotopic Mass | 406.97713 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 421 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
5-bromo-4-chloro-3-indolyl beta-D-galactoside is an indolyl carbohydrate that is the beta-D-galactoside of 3-hydroxy-1H-indole in which the indole moiety is substituted at positions 4 and 5 by chlorine and bromine, respectively. It is used to test for the presence of an enzyme, beta-galactosidase, which cleaved the glycosidic bond to give 5-bromo-4-chloro-3-hydroxy-1H-indole, which immediately dimerises to give an intensely blue product. It has a role as a chromogenic compound. It is an indolyl carbohydrate, a beta-D-galactoside, an organochlorine compound, an organobromine compound and a D-aldohexose derivative.
RELATED SUPPLIERS
Innovative
Mumbai
product:5-Bromo-4-Chloro-3-Indolyl-Beta-D-Galactopyranoside (X-Gal) (CAS Number: 7240-90-6)
