6754-58-1
Product Name:
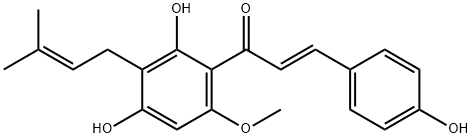
Xanthohumol
Formula:
C21H22O5
Synonyms:
(E)-1-[2,4-Dihydroxy-6-methoxy-3-(3-methyl-2-butenyl)phenyl]-3-(4-hydroxyphenyl)propenone;2′,4,4′-Trihydroxy-6′-methoxy-3′-prenylchalcone;2′,4,4′-Trihydroxy-6′-methoxy-3′-prenylchalcone
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Melting Point | 172 °C |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
COMPUTED DESCRIPTORS
| Molecular Weight | 354.4 g/mol |
|---|---|
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 354.14672380 g/mol |
| Monoisotopic Mass | 354.14672380 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 515 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Xanthohumol is a member of the class of chalcones that is trans-chalcone substituted by hydroxy groups at positions 4, 2' and 4', a methoxy group at position 6' and a prenyl group at position 3'. Isolated from Humulus lupulus, it induces apoptosis in human malignant glioblastoma cells. It has a role as a metabolite, an apoptosis inducer, an antineoplastic agent, an antiviral agent, an EC 2.3.1.20 (diacylglycerol O-acyltransferase) inhibitor and an anti-HIV-1 agent. It is a member of chalcones, a polyphenol and an aromatic ether. It is a conjugate acid of a xanthohumol(1-).
